Due in part to COVID-19, more and more people realize the importance of taking early steps to understand the virulence mechanisms of pathogens, especially in the face of their widespread resistance to drugs. Doing so gives researchers and clinicians a head start in preparing against future outbreaks, which means additional lives can be saved.
The laboratory of Robert Gennis, Professor Emeritus of Biochemistry in collaboration with the Thomas Kehl-Fie laboratory in the Department of Microbiology, recently released a paper titled “Role of respiratory NADH oxidation in the regulation of Staphylococcus aureus virulence” which provides some insight into those mechanisms within S. aureus.
Lici Schurig-Briccio, Research Scientist and first author on the paper, described the lab’s motivation to study this topic: “We chose S. aureus as our model system because it’s of great public health concern since it produces many life-threatening diseases and it can infect almost every place in the human body, like lungs, heart, and bone, producing different types of diseases. The pathogen is able to do this because it can adapt by choosing different metabolic pathways or even respiratory enzymes to optimize for survival in each environment. The respiratory enzymes are particularly important because they are directly involved in the energy supply to the cell as well as maintaining redox balance. Moreover, we specifically chose to study this pathogen because the composition of its respiratory (electron transport) chain is quite unique. Our main goal in this context is to understand what role that respiratory chain plays in S. aureus virulence.”
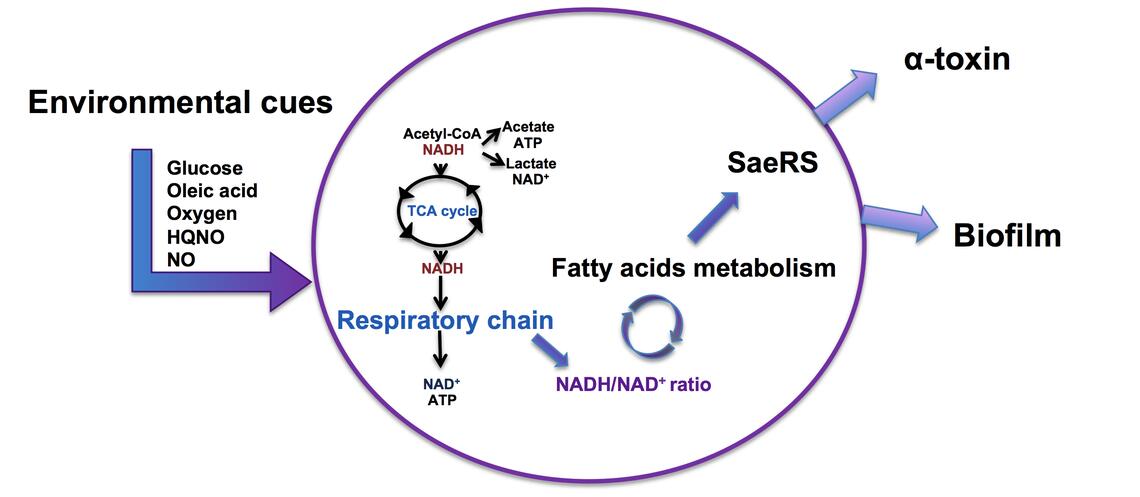
The lab studied the importance of Type II NADH dehydrogenases (NDH-2) within S. aureus both in vivo and in vitro to see how well the bacteria could grow and display traditional markers of virulence, such as production of alpha-toxin and pigment, and the formation of biofilm.
NDH-2s oxidize NADH, reduce menaquinone in the membrane, and pass electrons to cytochrome oxidases within the respiratory chain, which pump protons across the membrane and generate a membrane potential to allow the cell to produce ATP. Unlike many other bacteria, S. aureus has NDH-2s as their only respiratory enzymes that oxidize NADH, placing them in a unique position in the metabolism of this pathogen.
Mammalian mitochondria do not contain NDH-2s, whereas these enzymes are important in several pathogens (e.g. S. aureus, Streptococcus agalactiae, Toxoplasma gondii and Plasmodium falciparum). This observation initially directed the attention of Dr. Schurig-Briccio to these enzymes to evaluate their role in virulence.
“We were the first to look at the NDH-2s within S. aureus. This organism has two NDH-2s (NdhC and NdhF) which, despite apparent redundancy in their biochemical activity, each uniquely contribute to virulence.” Schurig-Briccio said.
S. aureus cells lacking NDH-2s can no longer directly link the oxidation of NADH to respiration and, therefore, suffer from a high NADH/NAD+ ratio which has large consequences to the bacterial metabolism. Because of this, cells lacking NDH-2s are less virulent.
“There are many things in the cell that are regulated by the NADH/NAD+ ratio. For example, the central metabolic tricarboxylic acid cycle requires NAD+ to keep working,” Schurig-Briccio said. “The redox imbalance resulting from not having NDH-2s stresses the bacteria which, through an unknown mechanism, causes the cells to accumulate free fatty acids.”
When the S. aureus cells accumulate free fatty acids, their virulence is inhibited by a two-component regulatory system (SaeRS), which senses environmental and intracellular signals as well as regulates the cells’ functions in response to those signals. Through regulation of major energy expenditures such as biofilm formation and production of virulence factors, the cells can adapt to any environment to survive.
Through those means, the SaeRS two-component system halts infection after sensing fatty acid accumulation from NADH/NAD+ imbalance within the cells, which in turn is caused by improper NDH-2 functioning.
“The SaeRS two-component system tells the cells, ‘Stop! Don’t make biofilm or alpha toxin right now because you don’t need it to survive.” Schurig-Briccio said.
She continued, “the mechanism by which S. aureus NDH-2s alter fatty acid metabolism has not been studied previously and it is part of our future plans. Furthermore, the significance of fatty acid homeostasis to virulence requires further clarification in both S. aureus and more generally.”
An imbalance of redox homeostasis is well known to be related to human diseases and ageing. It is important to realize that upsetting the redox homeostasis is also critical to the normal functioning of bacterial pathogens. Learning the specifics of how this works may open the door to figuring out how to manipulate the redox balance of S. aureus to reduce the ability of the cells to optimize its metabolism in different environments and, therefore, reduce virulence.
“The role of metabolic adaptation in S. aureus virulence was previously of less interest to researchers interested in virulence, because effective antibiotics were readily available. But now that there is a growing crisis due to drug resistance, these issues are important to find the next generation of drug targets. We need to try to understand how S. aureus and other bacterial pathogens survive in and adapt to all these different environments despite host resistance mechanisms,” Schurig-Briccio said.

