
When confronted with infection or injury, our body rallies to fight and heal itself and inflammation is a key part of this early defense system. But inflammation can also go awry, as exhibited in chronic illnesses such as obesity and type 2 diabetes. Biochemistry professor Lin-Feng Chen has spent years working to understand the regulation behind the immune system’s inflammatory response.
Scientists have known that NF-kB (nuclear factor kappa B) is an important transcription factor regulating innate and adaptive immune responses. In 2009, Chen and colleagues identified the protein BRD4 (Bromodomain-containing protein 4) as a co-activator, or partner, that works with NF-kB to regulate inflammatory gene expression. In the years since, researchers have studied BRD4 as a possible therapeutic target for treating cancer and inflammatory diseases.
“We’re trying to understand how BRD4 can facilitate NF-kB and other transcription factors to regulate inflammatory gene expression or the expression of other genes, including genes in cell proliferation and cell metabolism,” Chen said.
Most recently, his lab has identified the novel mechanism by which BRD4 can regulate the innate immune response, the body’s first line of defense, to bacterial infection. Results from the study, led by Nikita Modi, who received her PhD in biochemistry from Illinois earlier this year, were outlined in the article, “BRD4 regulates glycolysis-dependent Nos2 expression in macrophages upon H. pylori infection,” in CMGH, the journal of the American Gastroenterology Association.
For the study, the lab used a mouse model that had the BRD4 gene knocked out in specific immune cells such as macrophages. They then sought to analyze how inflammatory genes were induced by the infection of the gastric pathogen Helicobacter pylori, or H. pylori. More than 50 percent of the world’s population are believed to be infected with this gut bacteria. H. pylori infections can cause chronic inflammation in the stomach, which can lead to peptic ulcer disease and gastric cancer.
Macrophages are specialized immune cells that play a critical role in mediating the innate immune response by clearing infections and recruiting other immune cells to fight infection. Researchers hypothesized that BRD4 played a critical role in macrophages’ response to H. pylori infection.
BRD4, they learned, can regulate innate immune response through metabolic reprogramming of macrophages. Such programming is important for macrophage polarization, the process by which macrophages take on different roles for fighting and healing from infections. In response to various stimuli, macrophages can be directed to the M1 phenotype, adopting glycolysis to generate energy, activating specific inflammatory genes, and producing cytokines to eliminate pathogens. Alternatively, they can be reprogrammed to M2 phenotypes, employing oxidative phosphorylation for ATP production, contributing to tissue repair and resolving inflammation.
The lab has also identified HIF-1a as a new partner for BRD4 in the control of metabolic genes for energy production and regulating inflammatory gene activation. Their research also revealed that mice lacking BRD4 failed to eliminate H. pylori infection.
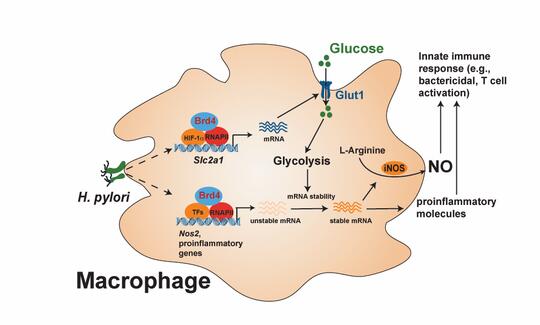
“BRD4 can basically regulate the HIF-1a-dependent glycolysis. This kind of glycolysis is found in cancer cells, which use glucose as a kind of energy source. … How BRD4 works with HIF-1a is not very clear. This is one of the things we’re trying to understand. Glycolysis is important for activation of macrophages upon H. pylori infection. We also found another function of this glycolysis. It can regulate the mRNA stability of the protein called iNOS, which mediates the production of nitric oxide, a chemical responsible for killing bacteria.”
Looking ahead, the lab is also planning to use the BRD4 knockout mouse models in combination with different mouse disease models to see how BRD4-mediated immune response contributes to different kinds of pathological states.
“Macrophages are not just good for fighting against infections, sometimes they do bad things. … We are using our mouse model to try to also understand the role of Brd4 in shaping the bad macrophages, including adipose tissues macrophages (ATMs) and tumor-associated macrophages (TAMs)," he said.
