
Contact Information
B103 CLSL
601 S Goodwin Ave
Urbana, IL 61801
Research Interests
Enzymology, Genetics, Membrane Biology, Microbial Physiology, Regulation of Gene Expression
Research Description
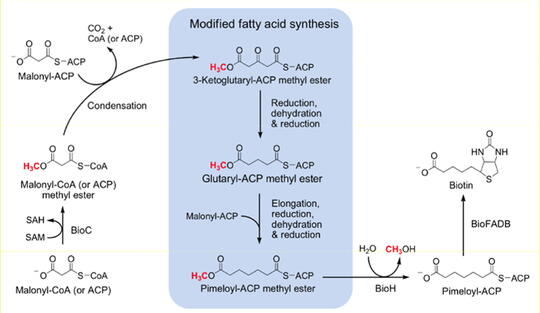
Genetic approaches to regulation of lipid metabolism; synthesis and attachment of biotin and lipoic acid
My laboratory works in two main areas-regulation of lipid metabolism and synthesis and attachment of fatty acid related enzyme cofactors. In both areas, the organism studied is Escherichia coli due to its sophisticated genetics and simple lipid composition. However we also study a variety of gram positive bacteria.
We have studied mechanisms that regulate the composition of the membrane phospholipids of E. coli and are currently concerned how this organism regulates lipid synthesis such that it is neither "fat nor lean." Although, E. coli is the paradigm for bacterial lipid synthesis there are bacteria that are missing genes that are in essential for E. coli growth. We are in process of figuring out which proteins these bacteria use in place of those used by E. coli. This has practical consequences because bacterial fatty acid synthesis is the target of several newly developed antibiotics. We have found that two key coenzymes, how lipoic acid and biotin, found in all three domains of life require the fatty acid synthetic pathway for their synthesis. We have determined how E. coli makes these coenzymes and are now expanding into the pathways found in other bacteria.
Education
B.A. (Biology), California State University, 1965
Ph.D. (Molecular Biology), University of California, Irvine, 1968
Postdoctoral (Biochemistry), Washington University School of Medicine, 1968-1970
Awards and Honors
Member, National Academy of Sciences
Fellow, American Academy of Microbiology
University Scholar
MERIT Award, NIH
Microbiology Alumni Endowed Professorship
Additional Campus Affiliations
External Links
Highlighted Publications
Representative Publications
Hu, Z and Cronan, J. E(2020) The primary step of biotin synthesis in mycobacteria. Proc. Natl Acad, Sci U S A 117:23794-23801. PMID 32900960. PMC7519262
Hu, Y. and Cronan, J. E(2020) α-Proteobacteria synthesize biotin precursor pimeloyl-ACP using the BioZ 3-ketoacyl-ACP synthase and lysine catabolism. Nature Commun;11::5598. PMCID: PMC7645780.
Hu,, Z., Ma,, J, Tong,, W., Zhu, L, Wang,, H. and J E Cronan (2021) Escherichia coli FabG 3-ketoacyl-ACP reductase proteins lacking the assigned catalytic triad residues are active enzymes. J. Biol Chem Feb 2;:000365. PMC7973133.
Dong, H and Cronan, J. E. (2021) Temperature regulation of membrane composition in the firmicute, Enterococcus faecalis, parallels that of Escherichia coli. Environ Microbiol 2021 May;23(5):2683-2691. doi: 10.1111/1462-2920.15512. Epub 2021 Apr 18.PMID: 33830615
Song, X. Henke, S. K. and Cronan, J. E. (2021) A division of labor between two biotin protein ligase homologs. Mol Microbiol May 24. doi: 10.1111/mmi.14761.
Huijuan Dong, H. Ma, J., Chen, Q., Chen, B, Liang, L., Liao, Y., Song, Y., Wang, H. and Cronan, J. E. (2021) A cryptic long.-chain 3-ketoacyl-ACP synthase in the Pseudomonas putida F1 unsaturated fatty acid synthesis pathway. J. Biol. Chem. Jun 25:100920. doi: 10.1016/j.jbc.2021.100920.
Cronan, J. E. (2020) Progress in the enzymology of the mitochondrial diseases of lipoic acid requiring enzymes. Front. Genet. 21;11:510. PMCID: PMC7253636
Cronan, J. E. (2021) The Escherichia coli FadR transcription factor: Too much of a good thing? Mol. Microbial Dec 7. doi: 10.1111/mmi.14663. Online ahead of print.
Sirithanakorn,, C. and Cronan, J. E. (2021) Biotin, an essential cofactor: synthesis, ligation, regulation and role in virulence. FEMS Microbiol Rev. Jan 11:fuab003. doi: 10.1093/femsre/fuab003. Online ahead of print. PMC7973133.
Cronan, J. E. (2021) The Classical, Yet Controversial, First Enzyme of Lipid Synthesis: Escherichia coli Acetyl-CoA Carboxylase. Microbial. Mol. Biol. Rev. un 16:e0003221. doi: 10.1128/MMBR.00032-21.
Recent Publications
Cronan, J. E. (2026). Approaches to Study Proteins Encoded by Essential Genes. Proteins: Structure, Function and Bioinformatics, 94(2), 463-476. https://doi.org/10.1002/prot.70039
Dong, H., Zou, Q., & Cronan, J. E. (2025). Defining the Enterococcus faecalis Fatty Acid Kinase System of Exogeneous Fatty Acid Utilization. Molecular Microbiology, 124(5), 400-412. Advance online publication. https://doi.org/10.1111/mmi.70017
Hang, X., Zhang, L., Huang, Y., Hu, Y., Zeng, Q., Cronan, J. E., Zhang, L., & Bi, H. (2025). Switching the strict substrate specificities of the β-ketoacyl-acyl carrier protein synthases, FabH and BioZ. Proceedings of the National Academy of Sciences of the United States of America, 122(45), Article e2509301122. https://doi.org/10.1073/pnas.2509301122
Zou, Q., Dong, H., & Cronan, J. E. (2025). Enterococcus faecalis requires unsaturated fatty acids to overcome toxicity of environmental saturated fatty acids. Microbiology (United Kingdom), 171(9), Article 001602. https://doi.org/10.1099/mic.0.001602
Zou, Q., Dong, H., & Cronan, J. E. (2025). Two putative Enterococcus faecalis fabG genes do not encode β-ketoacyl-acyl carrier protein reductases. Microbiology (United Kingdom), 171(9), Article 001610. https://doi.org/10.1099/mic.0.001610




