
Contact Information
417 Roger Adams Lab B-4
600 S Mathews Ave
Urbana, IL 61801
Research Interests
Research Topics
Ion Channels, Membrane Biology, Protein Dynamics, Receptor Biochemistry
Research Description
Protein-Lipid interactions; Membrane Fusion; Lipid Metabolism
Research in the Fratti lab focuses on how the chemical and physical properties of membrane bilayers control the function of proteins. The connection between lipids and protein function are important throughout biology and dysregulation is linked to a wide array of diseases including cancer, diabetes, and Alzheimer’s disease. Therefore, it is important that we understand the fundamentals of protein-lipid interactions so that we can better address their disruption in diseases.
We use lysosomes/vacuoles from the yeast Saccharomyces cerevisiae to reveal how specific lipids inhibit or promote different mechanisms in the process of membrane fusion catalyzed by SNARE proteins. Fusion events are often carried out in highly organized membrane platforms, collectively known as microdomains that are enriched in regulatory lipids that partition from bulk lipids (e.g., phosphatidylcholine) that make up a vesicle. Although low in concentration, regulatory lipids are critical in cellular signaling and the formation of membrane microdomains. The lipids that drive microdomain assembly include phosphatidic acid (PA), diacylglycerol (DAG), phosphoinositides (PI), cholesterol, and sphingolipids. The stoichiometry of these lipids is constantly changing through the action of lipid phosphatases, kinases, and lipases. Lipid modification and remodeling of membranes dramatically changes how lipids interact with proteins and can affect local physical properties such as curvature, fluidity, and bilayer stability. Thus, protein function can be regulated by changes by the lipids in a membrane. Using yeast vacuoles as well as synthetic vesicles we will determine how lipid modification regulates proteins on vesicles as they move through the different stages of the fusion pathway and how dysregulation of these events affects specific genetic and infectious diseases.
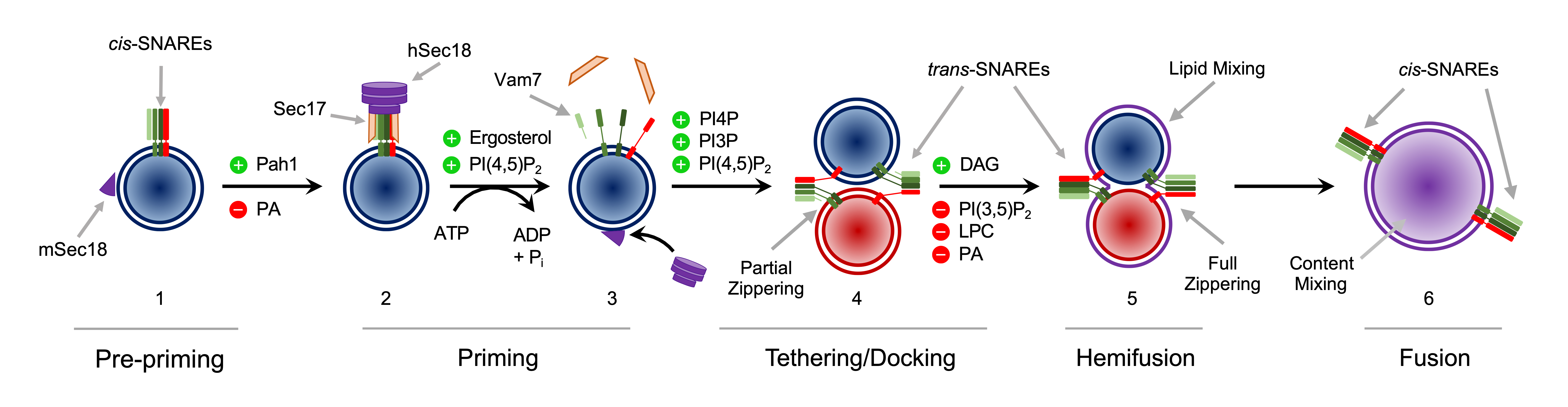
Figure 1. The Vacuole Fusion Cycle.
Depicted are the stages of vacuole fusion. 1) Isolated vacuoles contain inactive cis-SNARE complexes composed of the 3Q-SNAREs (Vam7, Vam3 and Vti1, shades of green) and 1R-SNARE (Nyv1, red). Also pictured is a PA-bound monomeric Sec18 (mSec18, purple wedge) associated with the membrane. Pah1 activity promotes moving to the next stage while excess PA blocks the progression. 2) Hexameric Sec18 (hSec18) associates with cis-SNARE through the use of Sec17 (orange trapezoids). This is promoted by ergosterol and PI(4,5)P2. 3) Sec18 hydrolyzes ATP to use Sec17 as a molecular wedge to separate the cis-SNARE bundle into individual proteins. Sec17 are and Vam7 released from the membrane. Individual SNAREs are shown to partially secondary structure in their SNARE motifs depicted as elongated lines extending from the ovals. Sec18 is thought to re-associate with the membrane as a PA-bound protomers. 4) During vacuole docking SNAREs interact in trans and partially zipper. This is promoted by PI3P, PI4P and PI(4,5)P2. 5) trans-SNARE complexes continue to fully zipper leading to hemifusion. The outer leaflets of both compartments merge (purple), while the inner leaflets remain separated to prevent content mixing. This is promoted by DAG, while it is inhibited by PI(3,5)P2, LPC and PA. 6) Inner leaflets fusion to fully merge the compartments into a continuous membrane and mixing luminal content (purple). The post-fusion SNAREs are shown now in cis and the vacuole returns to step 1 of the cycle. (See Starr & Fratti TIBS 2019)
Education
B.S. 1992 California State University, Long Beach
Ph.D. 2002 University of Michigan
Postdoc. 2002-2006 Dartmouth Medical School
Additional Campus Affiliations
Highlighted Publications
Zhang C, Calderin JC, Topiwalla A, Shah V, Karat JM, Knapp CT, Ahmed R, Grudzien D, Williamson EF, Fratti RA*. 2025. Vacuolar Phosphatidylinositol 3,4,5-trisphosphate controls fusion through binding Vam7, and membrane microdomain assembly. BioRxiv. https://doi.org/10.1101/2025.08.01.668199
Zhang C, Feng Y, Calderin JC, Balutowski A, Ahmed R, Knapp C, Fratti RA. 2024. Lysophospholipid headgroup size, and acyl chain length and saturation differentially affect vacuole acidification, Ca2+ transport, and fusion. BioRxiv
Zhang C, Calderin JD, Hurst LR, Gokbayrak ZD, Hrabak MR, Balutowski A, Rivera-Kohr DA, Kazmichuk TDD, Brett CL, Fratti RA. 2024. Sphingolipids containing very long-chain fatty acids regulate Ypt7 function during the tethering stage of vacuole fusion. J Biol Chem. DOI: 10.1016/j.jbc.2024.107808
Zhang C, Miner GE, Rivera-Kohr DA, Hrabak M, Balutowski A, Sullivan KD, Guo A, Feng Y, Calderin JD, Fratti RA. 2022. Interdependent transport of Ca2+ and H+ on yeast vacuoles and the role of Phosphatidylinositol 3,5-bisphosphate. J Biol Chem. DOI: https://doi.org/10.1016/j.jbc.2022.102672
Miner GE, Sullivan KD, Zhang C, Rivera-Kohr DA, Guo A, Hurst LR, Ellis EC, Starr ML, Jones BC, Fratti RA. 2020. Phosphatidylinositol 3,5-Bisphosphate regulates Ca2+ Transport During Yeast Vacuolar Fusion through the Ca2+ ATPase Pmc1. Traffic. 21:503-517.
Hurst LR, Fratti RA. 2020. Sphingolipids and Lipid Rafts in Yeast Vacuole Fusion and Maturation. Front Cell Dev Biol. 8:539.
Sparks R, Lui A, Bader D, Patel R, Murr M, Guida W, Fratti R, Patel N. 2019. A specific small-molecule inhibitor of Protein Kinase C-delta I activity improves metabolic dysfunction in human adipocytes from obese individuals. J Biol. Chem. 294, 14896-14910.
Sparks RP, Arango AS, Starr ML, Aboff ZL, Hurst LR, Rivera-Kohr DA, Zhang C, Harden KA, Jenkins JL, Guida WC, Tajkhorshid E, Fratti RA. 2019. A small-molecule competitive inhibitor of phosphatidic acid binding by the AAA+ protein NSF/Sec18 blocks the SNARE-priming stage of vacuole fusion. J Biol. Chem. 294, 17168-17185. * Featured Article
Starr ML, Sparks RP, Hurst LR, Zhao Z, Arango A, Lihan M, Jenkins JL, Tajkhorshid E, Fratti RA. 2019. Phosphatidic acid induces conformational changes in Sec18 protomers that prevent SNARE priming. J. Biol. Chem. 294:3100-3116.
Starr ML, Fratti RA. 2019. The Participation of Regulatory Lipids in Vacuole Homotypic Fusion. Trends Biochem. Sci. 44:546-554 . * Featured Article
Miner GE, Sullivan KD, Guo A, Jones BC, Hurst LR, Ellis EC, Starr ML, Fratti RA. 2019. Phosphatidylinositol 3,5-Bisphosphate Regulates the Transition between trans-SNARE Complex Formation and Vacuole Membrane Fusion. Mol. Biol. Cell. 30:201-208.
Starr ML, Hurst LR, Fratti RA. 2016. Phosphatidic acid sequesters Sec18p from cis-SNARE complexes to inhibit priming. Traffic. 17:1091-1109.
Miner G, Starr ML, Hurst LR, Sparks RP Padolina M, Fratti RA. 2016. The Central Polybasic Region of the Soluble SNARE (Soluble N-Ethylmaleimide-sensitive Factor Attachment Protein Receptor) Vam7 Affects Binding to Phosphatidylinositol 3-Phosphate by the PX (Phox Homology) Domain. J. Biol. Chem. 291:17651-17663.
Lawrence G, Flood B, Brown C, Karunakaran S, Cabrera M, Nordmann M, Ungermann C, Fratti RA. 2014. Dynamic association of the PI3P-interacting Mon1-Ccz1 GEF with vacuoles is controlled through its phosphorylation by the type-1 casein kinase Yck3. Mol. Biol. Cell. 25:1608-1619.
Sasser T, Karunakaran S, Qiu Q, Padolina M, Reyes A, Flood B, Smith S, Fratti RA. 2012 The Yeast Lipin 1 Orthologue Pah1p Regulates Vacuole Homeostasis and Membrane Fusion. J. Biol. Chem. 287:2221-2236.
Full List of Publications.
Recent Publications
Calderin, J. D., Zhang, C., Tan, T. J. C., Wu, N. C., & Fratti, R. (2025). Use of Bio-Layer Interferometry (BLI) to Measure Binding Affinities of SNAREs and Phosphoinositides. In Methods in Molecular Biology (pp. 103-117). (Methods in Molecular Biology; Vol. 2887). Humana Press Inc.. https://doi.org/10.1007/978-1-0716-4314-3_7
Fratti, R. (2025). Preface. Methods in Molecular Biology, 2887, v.
Fratti, R., Calderin, J. D., & Starr, M. L. (2025). Spectroscopic Methods for Detecting Conformational Changes During Sec18-Lipid Interactions. In Methods in Molecular Biology (pp. 119-132). (Methods in Molecular Biology; Vol. 2887). Humana Press Inc.. https://doi.org/10.1007/978-1-0716-4314-3_8
Malwal, S. R., Garcia-Rubio, R., Kordalewska, M., Patterson, H., Zhang, C., Calderin, J. D., Zhou, R., Pandey, A. M., Shor, E., Perlin, D. S., Wiederhold, N. P., Ostrosky-Zeichner, L., Fratti, R., Nacy, C., & Oldfield, E. (2025). Broad-Spectrum Activity and Mechanisms of Action of SQ109 on a Variety of Fungi. ACS Infectious Diseases, 11(6), 1662-1672. https://doi.org/10.1021/acsinfecdis.5c00210
Zhang, C., Calderin, J. D., Hurst, L. R., Gokbayrak, Z. D., Hrabak, M. R., Balutowski, A., Rivera-Kohr, D. A., Kazmirchuk, T. D. D., Brett, C. L., & Fratti, R. A. (2024). Sphingolipids containing very long-chain fatty acids regulate Ypt7 function during the tethering stage of vacuole fusion. Journal of Biological Chemistry, 300(11), Article 107808. https://doi.org/10.1016/j.jbc.2024.107808