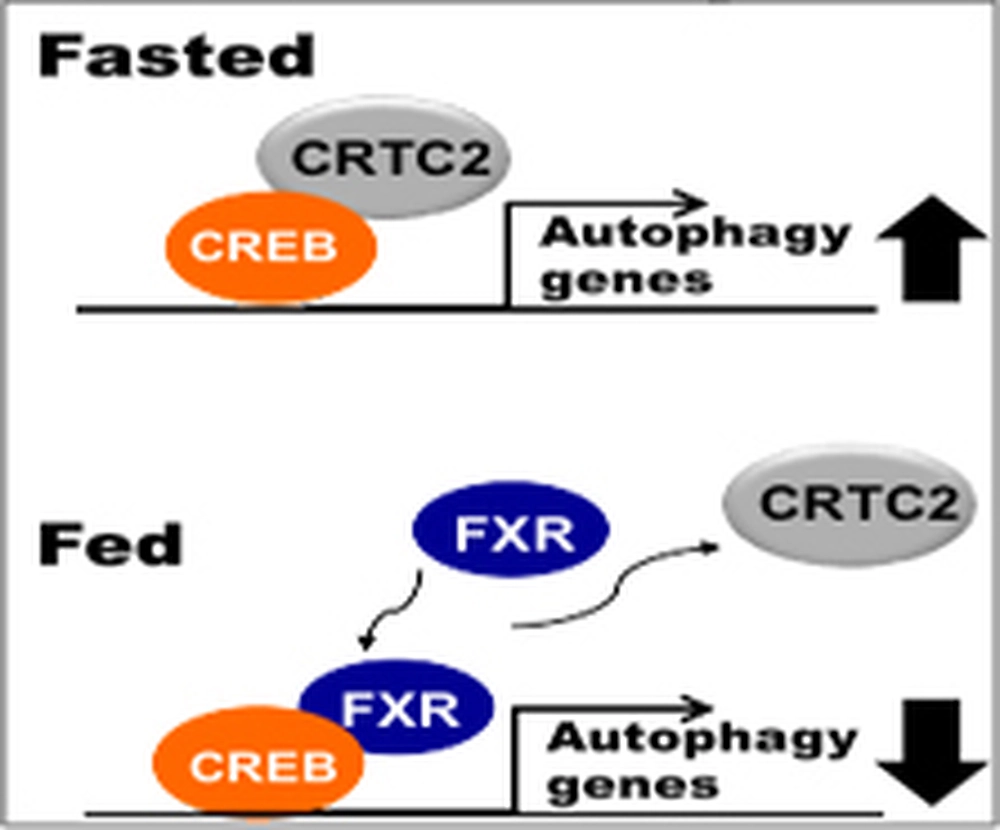
Autophagy or “self-eating” is the breakdown and recycling of cellular components and is essential for cellular survival under starvation but must be suppressed upon feeding. Acute regulation of preexisting autophagy machinery by protein phosphorylation is well defined, but longer-term regulation of the synthesis of these proteins is not. The team found that feeding-activated FXR and fasting-activated CREB are key physiological regulators of hepatic autophagy that oppositely regulate the autophagy gene network during feeding/fasting cycles.
Lysosomal degradation of cytoplasmic components by autophagy is essential for cellular survival and energy homeostasis under nutrient-deprived conditions. Acute regulation of autophagy by nutrient-sensing kinases, such as mTOR and AMPK, is well defined, but longer-term transcriptional regulation is not.
In this study, Sunmi Seok and Ting Fu (co-first authors) in the Jongsook Kim Kemper lab in the Department of Molecular and Integrative Physiology and collaborators, including Jian Ma’s group in the Department of Bioengineering, have identified the feeding-activated nuclear receptor FXR and the fasting-activated transcriptional factor CREB as key physiological regulators of autophagy.
There are several important aspects to this study. First, it was believed that autophagy occurs only under extremely stressful nutrient-deprived conditions but we show that it actually occurs physiologically during feeding/fasting. Second, autophagy does have important metabolic functions, for example lipophagy is dynamically regulated by the FXR/CREB axis. Third, this is the first report that FXR and CREB are transcriptional regulators of the autophagy gene network. Fourth, defective autophagy has been implicated in many diseases but excess autophagy is also harmful because it promotes cell death and also may provide a favorable microenvironment for tumor growth.
The team's discovery that the FXR/CREB axis tightly regulates the autophagy gene network reveals potential new targets for developing new drugs to treat human diseases associated with autophagy dysfunction, including metabolic disorders, neurodegenerative disease, and cancer. This study was supported by grants from National Institutes of Health to Professor Kemper.