Contact Information
601 S. Goodwin Ave.
Urbana, IL 61801
Research Interests
Research Topics
Genetics, Host-Pathogen Interactions, Microbial Physiology, Protein-Nucleic Acid Interactions, Regulation of Gene Expression, RNA Biology, Virology
Disease Research Interests
Infectious Diseases
Research Description
Mechanisms and applications of bacterial viruses (phages) and the immune systems targeted against them
Research in the Hatoum-Aslan lab seeks to gain a mechanistic understanding of the perpetual microscopic war that has been raging for billions of years between bacteria and the viruses that infect them. Bacterial viruses, also known as phages, attach to a specific host, inject their DNA, and replicate exponentially in a process that typically kills the host. In response, bacteria have evolved a myriad of complex immune systems to fend off invading phages. Our research uses the tools of molecular biology, genetic engineering, and biochemistry to probe and understand the defensive and offensive molecular mechanisms that bacteria and phages use against each other. A second research focus seeks to leverage the antimicrobial properties of phages and genetically engineer them for use in novel therapeutics. These efforts will not only help addresses the global health care crisis incited by the rise in antibiotic-resistant infections, but will also enable the design of phage-based tools that will enable microbiome engineering. We are also interested in exploring novel genome editing technologies and other potential applications for bacterial immune systems such as CRISPR-Cas.
Investigating mechanisms of CRISPR-Cas immunity in staphylococci
CRISPR-Cas systems are a class of prokaryotic immune systems that use small CRISPR RNAs (crRNAs) and CRISPR-associated (Cas) proteins to detect and destroy mobile genetic elements such as plasmids and invading phages. CRISPR-Cas systems are remarkably diverse, with two broad classes and six distinct Types currently described. Type III systems are among the most widespread in nature, and likely the most complex. We study a model Type III CRISPR-Cas system found in Staphylococcus species, also known as CRISPR-Cas10. Our research has significantly contributed to our understanding of how CRISPR-Cas10 leverages cellular processes previously considered unrelated to immunity (Walker et al, 2017; Chou-Zheng and Hatoum-Aslan, 2019, 2022) and how phages have evolved to overcome immunity (Chou-Zheng and Howell et al, 2024).
Engineering phage-based diagnostics and antimicrobials
The spread of antibiotic resistance in pathogenic bacteria, coupled with the sharp decline in the discovery of new antibiotics, are together responsible for inciting a global public health crisis. Drug-resistant staphylococci are leading causes of healthcare-associated infections, and virulent phages, which kill staphylococci within minutes of infection, represent viable alternatives or supplements to conventional antibiotics. However, phage genomes are replete with genes of undetermined functions which can cause harmful downstream side-effects. Our work aims to help stem regulatory and safety concerns over the routine use of phages in humans. To achieve this, we developed a platform for the genetic engineering of virulent phages using CRISPR-Cas10 (Bari et al, 2017). Ongoing work seeks to engineer phages with well-defined components that can potentially be used as diagnostic tools and alternatives to antibiotics for the treatment of drug-resistant infections.
Discovering and characterizing new phages and anti-phage immune systems
Phages are considered the most abundant entities on the planet, with an estimated 1031 particles in the biosphere; However, a detailed understanding of their diversity and lifestyles remains in its infancy. Ongoing projects in the lab and in Dr. Hatoum-Aslan’s Research Experience in Microbiology course seek to discover and characterize new phages that infect pathogenic Staphylococcus and Streptococcus species. Dozens of phages have already been discovered (see Cater et al, 2017, for an example), and the continual expansion of our phage collection is paramount to understanding not only phage diversity, but also the diverse immune systems targeted against them. As an example, using our unique collection of S. epidermidis phages, we have uncovered a new immune system unlike any other described to date (Bari et al, 2022). Ongoing work continues to discover and characterize new phages and new defense systems, particularly in bacterial pathogens.
Promoting interest in science through education and outreach
Our group has also been engaged in synergistic educational activities that provide research experiences and active learning opportunities for undergraduates and underrepresented groups in STEM disciplines, with a goal to inspire the next generation of scientists to enter into STEM careers. Such activities include developing and implementing a semester-long research-based lab course for undergraduates called MCB 493-REM Research Experience in Microbiology, and a five-day educational module/kit titled Exploring the CRISPR-Cas defense system for high school students. Thus far, MCB 493-REM and its predecessor offered at the University of Alabama (called Phage Discovery) has provided over 200 undergraduates with authentic research experiences while discovering dozens of new phages, many of which have formed the basis for ongoing research projects in the lab.
Education
B.S. 1998 Florida Institute of Technology (Molecular Biology)
M.S. 2001 American University of Beirut (Biochemistry)
Ph.D. 2007 Cornell University (Biochemistry)
Postdoc. 2007-2008 Cornell University (Biochemistry)
Postdoc. 2010-2014 Rockefeller University (Bacteriology)
Awards and Honors
NIH/NIAID K22 Career Development Award, 2015
NSF/MCB CAREER Award, 2018
University of Alabama President's Faculty Research Award, 2018
Burroughs Wellcome PATH Award, 2020
Additional Campus Affiliations
Associate Professor, Microbiology
Affiliate, Carl R. Woese Institute for Genomic Biology
External Links
Highlighted Publications
Representative Publications
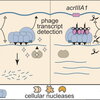
Chou-Zheng L, Howell O, Boyle TA, Hossain M, Walker FC, Sheriff EK, Aslan B, and Hatoum-Aslan A. (2024) AcrIIIA1 is a protein-RNA anti-CRISPR complex that targets core Cas and accessory nucleases. Nucleic Acids Res, 52(22): 13490-13514.
Hossain M, Aslan B, and Hatoum-Aslan A. (2024) Tandem mobilization of anti-phage defenses alongside SCCmec elements in staphylococci. Nat Comm, 15(1):8820.
Boyle TA and Hatoum-Aslan, A. (2023) “Recurring and emerging themes in prokaryotic innate immunity.” Curr Opin Micro, 73:102324
Bari SMN, Chou-Zheng L, Howell O, Hossain, Hill CM, Boyle TA, Cater K, Dandu VS, Thomas A, Aslan B, and Hatoum-Aslan A. (2022) A unique mode of nucleic acid immunity performed by a multifunctional bacterial enzyme. Cell Host & Microbe, 30: 1-13.
Chou-Zheng, L and Hatoum-Aslan A. (2022) “Critical roles for ‘housekeeping’ nucleases in Type III CRISPR-Cas immunity.” eLife, 11:e81897.
Hatoum-Aslan A. (2021) The phages of staphylococci: Critical catalysts in health and disease. Trends in Microbiology, 29(12), 1117-1129.
Chou-Zheng L and Hatoum-Aslan A. (2019) A Type III-A CRISPR-Cas system employs degradosome nucleases to ensure robust immunity. eLife, 8:e45393.
Nasef M, Muffly MC, Beckman AB, Rowe SJ, Walker FC, Hatoum-Aslan A*, and Dunkle JA*. (2019) Regulation of cyclic oligoadenylate synthesis by the S. epidermidis Cas10-Csm complex. RNA, 25(8), 948-962.
Hatoum-Aslan A. (2018). Phage Genetic Engineering Using CRISPR–Cas Systems. Viruses, 10(6), 335. DOI: 10.3390/v10060335.
Bari SMN, Walker FC, Cater K, Aslan B, and Hatoum-Aslan A. (2017). Strategies for editing virulent staphylococcal phages using CRISPR-Cas10. ACS Synth Biol, DOI: 10.1021/acssynbio.7b00240.
Cater K, Dandu VS, Bari SMN, Lackey K, Everett GFK, and Hatoum-Aslan A. (2017). A novel Staphylococcus podophage encodes a unique lysin with unusual modular design. mSphere, 22(2) e00040-17.
Walker FC, Chou-Zheng L, Dunkle JA, and Hatoum-Aslan A. (2017). Molecular determinants for CRISPR RNA maturation in the Cas10-Csm complex and roles for non-Cas nucleases. Nucleic Acids Research, 45(4), 2112-23.
Samai P, Pyenson N, Jiang W, Goldberg G, Hatoum-Aslan A, and Marraffini LA. (2015). Cotranscriptional DNA and RNA cleavage during type III CRISPR-Cas immunity. Cell, 161(5), 1164-74.
Recent Publications
Chou-Zheng, L., Howell, O., Boyle, TA., Hossain, M., Walker, FC., Sheriff, EK., Aslan, B., & Hatoum-Aslan, A. (2024). AcrIIIA1 is a protein–RNA anti-CRISPR complex that targets core Cas and accessory nucleases. Nucleic acids research, 52(22), 13490-13514. Article gkae1006. https://doi.org/10.1093/nar/gkae1006
Hill, C. M., & Hatoum-Aslan, A. (2024). Genetic Engineering of Therapeutic Phages Using Type III CRISPR-Cas Systems. In Methods in Molecular Biology (pp. 279-299). (Methods in Molecular Biology; Vol. 2734). Humana Press Inc.. https://doi.org/10.1007/978-1-0716-3523-0_18
Hossain, M., Aslan, B., & Hatoum-Aslan, A. (2024). Tandem mobilization of anti-phage defenses alongside SCCmec elements in staphylococci. Nature communications, 15(1), Article 8820. https://doi.org/10.1038/s41467-024-53146-z
Boyle, T. A., & Hatoum-Aslan, A. (2023). Recurring and emerging themes in prokaryotic innate immunity. Current Opinion in Microbiology, 73, Article 102324. https://doi.org/10.1016/j.mib.2023.102324
Paraan, M., Nasef, M., Chou-Zheng, L., Khweis, S. A., Schoeffler, A. J., Hatoum-Aslan, A., Stagg, S. M., & Dunkle, J. A. (2023). The structure of a Type III-A CRISPR-Cas effector complex reveals conserved and idiosyncratic contacts to target RNA and crRNA among Type III-A systems. PloS one, 18(6), Article e0287461. https://doi.org/10.1371/journal.pone.0287461