
In a new finding published in Nature Chemical Biology, Research Scientist Gisela Cymes and Associate Professor of Molecular and Integrative Physiology, Biophysics, and Neuroscience Claudio Grosman applied single-molecule electrophysiology to elucidate the properties of the ring of acidic side chains that catalyzes the flow of cations through the nicotinic acetylcholine receptor channel.
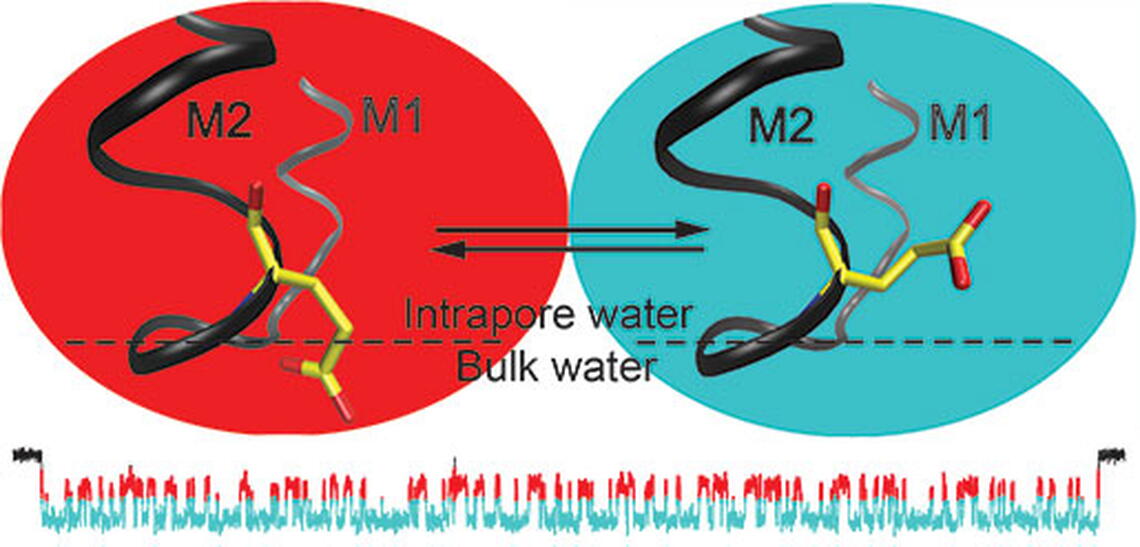
In a new finding published in Nature Chemical Biology, Research Scientist Gisela Cymes and Associate Professor of Molecular and Integrative Physiology, Biophysics, and Neuroscience Claudio Grosman applied single-molecule electrophysiology to elucidate the properties of the ring of acidic side chains that catalyzes the flow of cations through the nicotinic acetylcholine receptor channel. In contrast to what had been assumed for almost 25 years, Cymes and Grosman found that only two of the four glutamates in the selectivity filter of the muscle-type receptor contribute to the single-channel conductance, and that side-chain torsional flexibility and the properties of the microenvironment have a previously unrecognized role on ion permeation. As the authors put it, "This study reveals that the catalytic ring of acidic side chains forms a system of much higher complexity than could be anticipated from this channel's extremely simple conductance behavior. Indeed, not only the protonation state but also the location along the long axis of the pore and the conformation of these side chains seem to be key determinants of the cation-conduction free-energy profile".