Third-year Biophysics graduate student Tyler Harpole and Professor of Molecular and Integrative Physiology, Biophysics and Neuroscience, Claudio Grosman, have used molecular dynamics and Brownian dynamics computer simulations to test a novel hypothesis as to how the nicotinic acetylcholine receptor controls the rate at which cations enter the cell through the receptor’s transmembrane pore.
This influx of cations is the first step in a series of events that culminate in, for example, muscle contraction or neurotransmitter release, and its rate is tightly regulated. Indeed, naturally occurring mutations that slow down or speed up this flow of ions lead to disease.
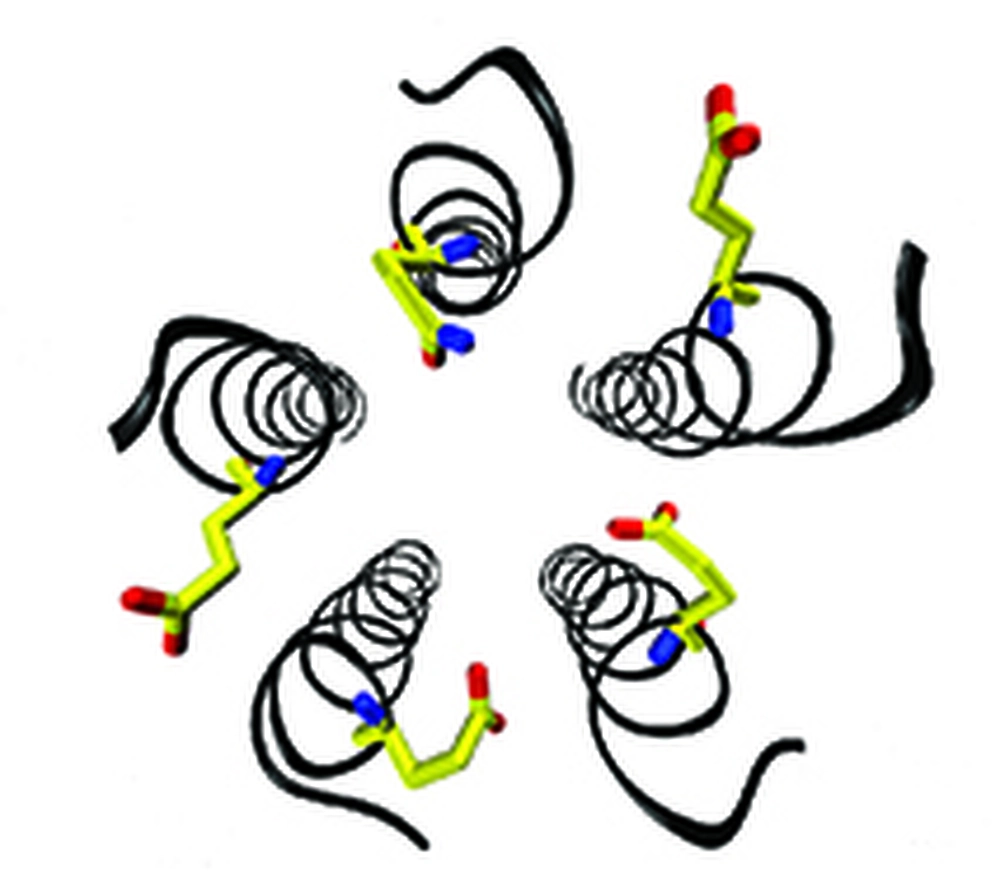
Previous mutagenesis work from the Grosman lab on the ring of glutamates in the charge-selectivity filter region of the muscle nicotinic receptor led them to propose that the rate at which ions permeate depends not only on the number of these glutamates, but also, on the conformation of their side chains. Because these inferences were made on the basis of electrophysiological observations, however, they decided to test the plausibility of these ideas using molecular simulations, thus taking advantage of the atomic detail and high temporal resolution that only these computational methods can provide. Remarkably, the simulations gave ample credence to all aspects of their proposal and allowed them to gain insight into the effect of specific glutamate rotamers on single-channel conductance.