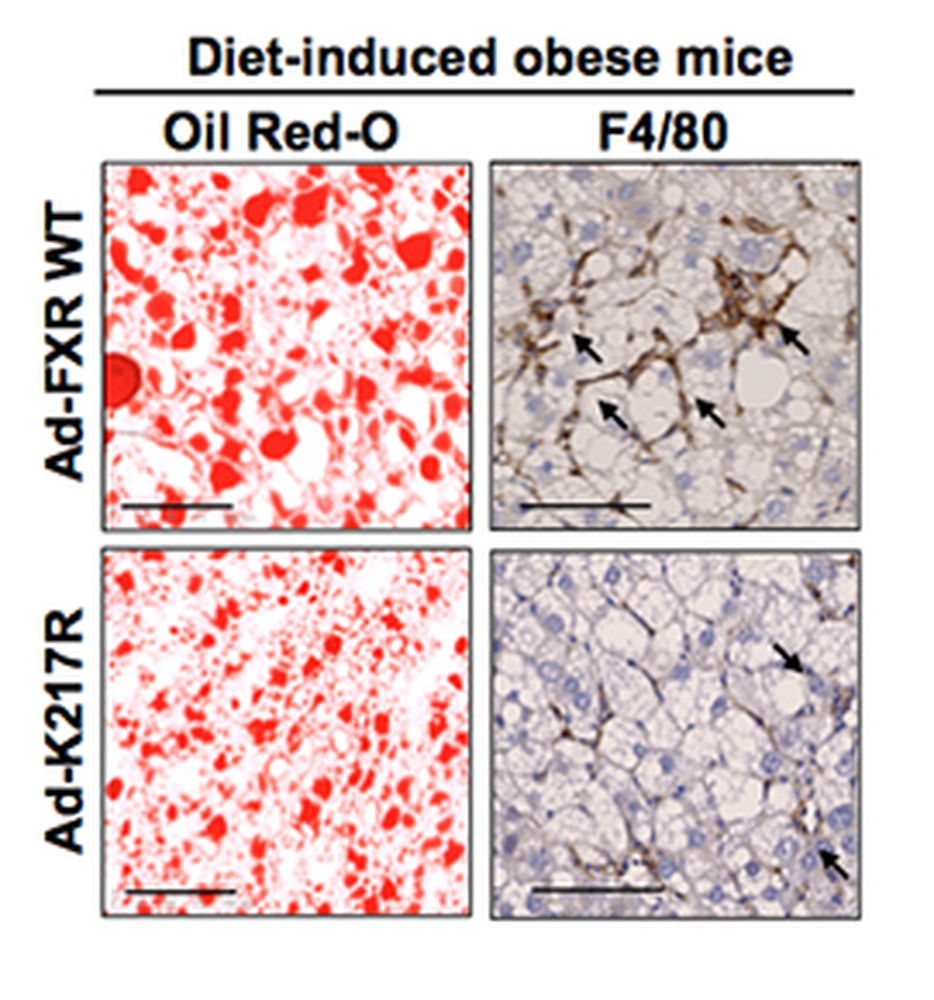
Associate Professor of Molecular and Integrative Physiology, Jongsook Kim Kemper, post-doctoral researcher, Dong-Hyun Kim, and their colleagues discovered that the function of a key metabolic transcriptional regulator, FXR, is modulated by an acetyl/SUMO switch, which is dysregulated in obesity. Elevated acetylation of FXR at Lys-217 in diet-induced obese mice inhibits its SUMOylation at Lys-277, which promotes hepatic inflammation and metabolic dysfunction. The findings are published in the EMBO Journal.
Posttranslational acetylation of transcriptional regulators is often persistently elevated in nutrient-excessive obesity conditions. In this study, we investigated the functional consequences of such elevated acetylation of the key metabolic regulator and bile acid nuclear receptor FXR.
Proteomic studies identified Lys-217 as the FXR acetylation site in diet-induced obese mice. In vivo studies utilizing acetylation-mimic and acetylation-defective Lys-217 mutants and gene expression profiling revealed that FXR acetylation increased proinflammatory gene expression, inflammatory responses, and liver fat levels, and impaired insulin sensitivity. Mechanistically, agonist-activated FXR is SUMO2-modified at lysine 277 by the PIASy SUMO E3 ligase. SUMOylation of FXR inhibits inflammatory gene expression in response to inflammatory signaling. Selective transrepression of inflammatory genes results from increased interaction of SUMO-FXR with NF-kB. In obese mice, FXR acetylation at lysine 217 inhibits its SUMOylation and diminishes SUMO2-dependent FXR anti-inflammatory action.
There are several important aspects to this study. First, although posttranslational modifications (PTMs) of proteins have been extensively studied, there is little evidence establishing the physiological/pathological relevance of PTMs and the mechanisms underlying PTM-mediated selective gene regulation. The present study, for the first time, demonstrates the relevance in vivo of a functional antagonism between acetylation and SUMOylation of FXR in diet-induced obese mice. Second, agonist-activated SUMOylated FXR selectively trans-represses NF-κB target inflammatory genes without transcriptionally regulating conventional FXR/RXRα target genes and further, the results indicate that the underlying mechanism is the selective interaction of SUMO2-FXR with NF-κB and not with RXRα. Third, since FXR is expressed in many non-hepatic tissues, including intestine, kidney, endothelial cells, and macrophages, the dysregulated acetyl/SUMO switch identified for liver FXR in obesity may also play a role in the pathogenesis of inflammatory diseases in other tissues, such as inflammatory bowel disease and atherosclerosis, as well as, liver steatohepatitis. Targeting the dysregulated acetyl/SUMO switch of FXR may provide novel therapeutic options and diagnostic markers for obesity-related inflammatory and metabolic disorders.
This study was supported by grants from National Institutes of Health to Professor Kemper and a post-doctoral fellowship from America Heart Association to Dong-Hyun Kim.