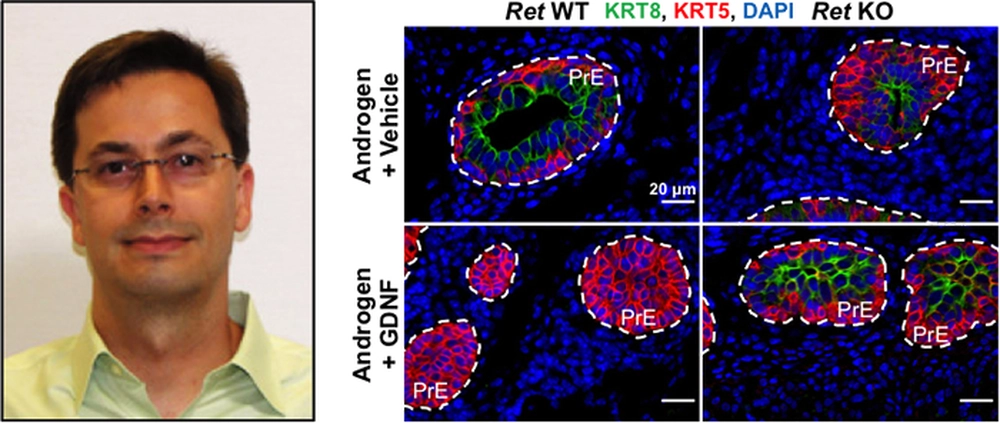
A new study published in Development by Assistant Professor Eric Bolton and Research Associate Hyun-Jung Park reveals that glial cell line-derived neurotrophic factor (GDNF), which signals through activation of RET tyrosine kinase, inhibits androgen-induced development of the mouse prostate gland.
The prostate gland is formed from the embryonic urogenital sinus, where the urethra joins the bladder. The androgen receptor (AR) is required for prostate development, and AR signaling acts in concert with other signaling pathways to orchestrate the proliferation and differentiation of mesenchymal and epithelial cells of the developing prostate. Importantly, the disruption of AR-mediated prostate development predisposes humans and rodents to prostate neoplasia by altering the gland’s phenotype early in life.
The study by Park and Bolton demonstrates that RET-mediated GDNF signaling in the mouse urogenital sinus increases proliferation of mesenchymal cells and suppresses androgen-induced proliferation and differentiation of prostate epithelial cells, thereby inhibiting prostate development. The study also establishes novel reciprocal regulatory crosstalk between AR and GDNF signaling in the cellular and molecular mechanism of androgen-induced prostate development. The researchers propose that the reciprocal regulatory crosstalk may serve to balance GDNF-induced proliferation of mesenchymal cells with androgen-induced proliferation and differentiation of prostate epithelial cells, which are mediated by AR signaling in mesenchymal cells. Thus, if the GDNF signaling pathway is not downregulated early in prostate development, elevated levels of GDNF signaling may lead to disruption of AR-mediated prostate development and increased risk for prostate neoplasia.
The National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health and the Campus Research Board at the University of Illinois funded this research.
