
Nearly two percent of pregnant women will face recurrent miscarriages, defined as the spontaneous loss of three or more consecutive pregnancies. Of that two percent, half of those miscarriages cannot be explained. Scientists assume genetic factors may play a role, but to date they have not been able to describe why those miscarriages occur.
To combat this issue, University of Illinois researchers Milan Bagchi and Indrani Bagchi have taken an important step toward revealing critical molecular mechanisms for pregnancy. Their article in PNAS, “A hypoxia-induced Rab pathway regulates embryo implantation by controlled trafficking of secretory granules,” provides a new understanding of embryonic implantation within the uterus, potentially leading to more effective management of future miscarriages.
“We had known for a long time that the uterine environment is hypoxic during early pregnancy in mammals,” said Indrani Bagchi, professor and Billie Field Endowed Chair in the College of Veterinary Medicine. “We knew that it happens, but we didn’t know why it happens or how the mother adapts to this low oxygen tension. The physiology was known, but the mechanism was not clear,” she said.
Since the uterus is a closed environment, the embryo encounters a hypoxic environment during early pregnancy as it travels through the fallopian tube to enter the uterine cavity. This process takes eight to nine days to occur in humans, after which the embryo implants itself into the uterine wall by breaching the uterine epithelial cell lining. If implantation is successful, new blood vessels form within the uterine tissue to redirect blood, oxygen, and nutrients to the growing embryo to sustain the pregnancy. If the embryo fails to anchor itself to the uterine wall or gain access to oxygen within a certain amount of time, then the mother loses the pregnancy.
“Normally we would think that oxygen is essential for any normal tissue function, but the fact of life is that many of our tissues operate under hypoxic conditions,” said Milan Bagchi, Deborah Paul Endowed Professor of Molecular and Integrative Physiology in the School of Molecular and Cellular Biology. “Hypoxia typically happens when the cells divide rapidly and tissue mass grows. The steroid hormones estrogen and progesterone act as conditioning mechanisms for pregnancy because as their levels go up in the woman’s body, they help grow the uterus in size and bulk in preparation for pregnancy. This process of rapid cell proliferation absorbs much oxygen and creates a huge metabolic demand, creating a hypoxic environment within the uterus.”
To survive in these low-oxygen environments, tissues must adapt by evolving cellular mechanisms that allow them to overcome their lack of oxygen so they can continue their biological functions. Therefore, successful implantation depends on the mother’s adaptive response to the embryo’s hypoxic environment.
Although uterine physiology during the course of pregnancy is well-characterized, scientists lacked a central molecular mechanism for the mother’s adaptive response during pregnancy. The newly published paper provides such a mechanism, along with details on how the uterine secretory pathway plays a central role in this process.
The labs found that in response to hypoxia, the maternal tissue expresses hypoxia-inducible factor 2 alpha (HIF2α), a transcription factor which binds to the promoter of Rab27—an important GTPase within the Rab superfamily—to promote the secretion of secretory granules containing critical factors, MMP-9 (matrix metalloproteinase) and VEGF (vascular endothelial growth factor). Rab27 plays a key role in protein trafficking as it guides the secretory granules and vesicles from the interior of the cell to the edge of the plasma membrane for secretion.
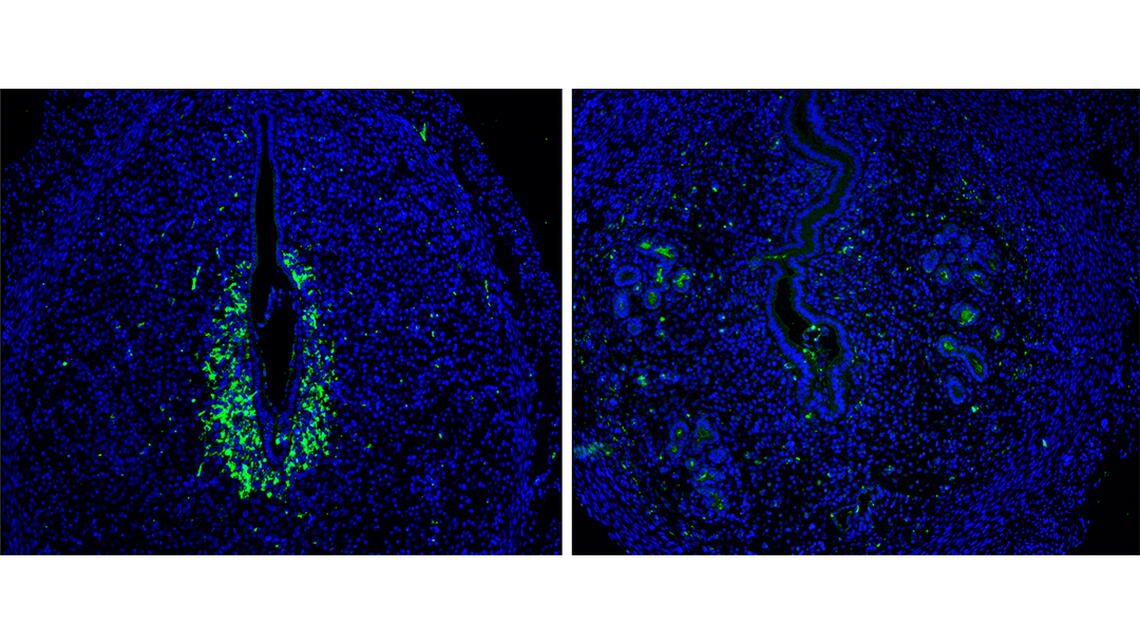
Once secreted outside the cell, MMP-9 directs tissue remodeling by breaking down the cellular matrix of the uterine epithelium and allowing the embryo to implant itself into the uterine wall. Secreted VEGF acts on endothelial (blood) cells, causing them to proliferate and form an extensive vascular network to redirect oxygen to the implanted embryo. If VEGF secretion is impaired, then the pregnancy will be lost since the embryo cannot survive without the support of a newly-formed vasculature.
Until now, scientists had considered hypoxia-inducible factor 1 alpha (HIF1α)—a ubiquitously-expressed factor present throughout pregnancy—to be a critical component of the implantation process. However, Milan and Indrani Bagchi were surprised to find that in knockout mice in which HIF2α was missing and HIF1α was still present, the embryo could not be sustained.
“Honestly, we did not expect the HIF2α mutant mice to display such a dramatic infertility phenotype. When we initially saw that HIF2α was induced at the time of implantation, we were just curious, thinking, ‘Maybe HIF2α has a role in implantation.’ If we had not seen this remarkable phenotype in the mutant mouse model, we would have assumed that HIF1α is the critical factor in the establishment of pregnancy instead of HIF2α,” Indrani Bagchi said.
In subsequent work, the labs continue to explore further and expand on the mechanism, even finding that the HIF2α-Rab27 pathway controls the trafficking of other important cellular vesicles such as exosomes. Having “stumbled” upon this important regulatory pathway, the labs believe that it will help them understand the crucial missing links in existing knowledge about implantation all the way up to the establishment and maintenance of pregnancy.
“It is extremely critical to understand the developmental biology and physiology of embryology, since this is how we learn how an embryo gets implanted and then grows within the womb. Without the successful execution of these critical processes, there is no pregnancy, and without pregnancy, there is no you or me,” Milan Bagchi said.
