As the first bacterium to be labeled a Group I carcinogen, Helicobacter pylori is the single most important risk factor for developing gastric cancer. The bacterium chronically infects over 50 percent of the world’s population and is estimated to be the third leading cause of cancer-related deaths in the world. Yet the mechanisms by which the protein factors produced by H. pylori contribute to bacterial infection and increase cancer risk remain poorly understood.
In a recent publication, the lab of Steven Blanke, Professor of Microbiology and Ralph S. Wolfe Professorial Scholar, has introduced a new approach for observing the effects of the microbe’s single protein toxin, called the vacuolating cytotoxin, or VacA, in the absence of infection. This is an important step towards determining the role of H. pylori’s toxin during gastric infection. The lab’s findings were published in a Nature Scientific Reports article entitled, “Chronic in vivo exposure to Helicobacter pylori VacA: Assessing the efficacy of automated and long-term intragastric toxin infusion.”
Blanke explained that bacterial toxins can sometimes be thought of as protein-based drugs that bacteria make and release to modulate the activity of host cells. In the case of H. pylori, the stomach is a harsh and hostile place for bacteria to establish an infection, considering the harsh acidic and harsh gastric environment. VacA had earlier been demonstrated, using animal models, to promote establishment of H. pylori in the not-so-friendly confines of the stomach environment, although the underlying mechanisms had not been established. Indeed, it’s almost always challenging to nail down the specific role of bacterial toxins during bacterial infection. This study conducted in the Blanke lab described an attempt to experimentally identify VacA-mediated changes during H. pylori infection, which can be attributed to the toxin’s action alone.

Scientists have longed believed that VacA contributed to the pathogenicity of H. pylori by directly destroying stomach cells. “When people discovered that H. pylori made VacA twenty years ago, they ground up bacteria, and introduced the extracts directly to the stomachs of animals, and found that the extracts caused a lot of damage. When they saw that, they ascribed that damage to the toxin,” Blanke said.
However, these early studies had a number of limitations, including the absence of studies that take into account the chronic nature of H. pylori infection, which can persist for years prior to the emergence of stomach disease, including cancer. The consequences of long-term exposure to carcinogens are not typically manifested in days or weeks, but perhaps only after years of exposure. Notably, children are particularly vulnerable to H. pylori infection early in life, but stomach cancer typically emerges in older adults. The single doses of bacterial extract administered during early animals studies likely did not replicate the effects of long-term infection and exposure to VacA. Therefore, the challenge has been to identify changes attributable to VacA over a long period of time, and in the absence of the bacteria, which typically synthesize and deliver the toxin directly in the stomach.
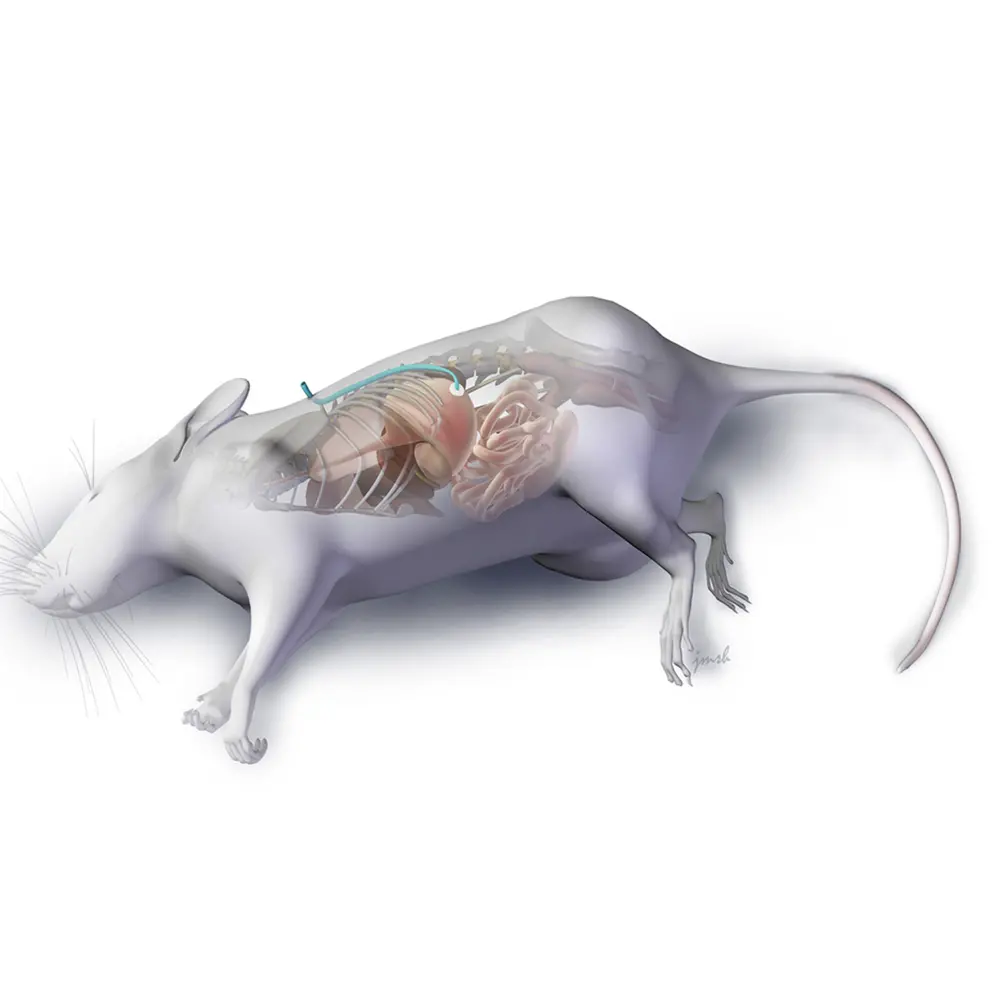
To mimic the process of VacA delivery during chronic bacterial infection, the Blanke lab implemented a novel method of administering purified toxin for long periods of time into the stomachs of mice. Designed and led by the paper’s first author Dr. Robin Holland, special G-tubes, or feeding tubes, were inserted into the mice’s stomachs and kept in place by jackets to prevent the mice from gnawing on them. The G-tubes continuously infused the stomachs, over the span of a month, with dilute amounts of VacA, in the absence of H. pylori, to mimic the steady, low stream of toxin secreted by bacteria during normal infection.
To their surprise, the lab found that VacA causes less damage to stomach cells than previous papers had reported. However, they found alterations in the stomach, suggesting that the toxin transforms the stomach from a hostile environment into a more suitable niche for long-term bacterial infection. In the same way that tobacco does not directly cause lung cancer but rather increases its risk by modifying the lungs, the changes specifically attributable to VacA both promote H. pylori infection and create an environment associated with a higher risk for carcinogenesis. Specifically, the animals infused with purified VacA demonstrated a thinning of the mucous layer that is normally protective for the stomach lining, and, induced changes in the stomach cells that release the acid within the stomach, which ultimately leads to neutralization of the stomach pH. Notably, both of these changes have been previously reported during long-term infection with H. pylori.
“When people first hear VacA increases stomach pH, they think, ‘Wait a minute, that’s a good thing, right?’ because they take Tums or proton pump inhibitors when they get heartburn. In the short term, that works out fine, but it’s bad in the long term,” Blanke said. “For example, in some animal models, long-term neutralization of stomach acidity can alone lead to stomach cancer. Therefore, raising the stomach’s pH in humans over time profoundly changes the organ, eventually leading it to lose its ability to function as a stomach and regulate hormones like it’s supposed to. In fact, increasing the pH can lead to atrophy of the stomach, intestinal metaplasia, a process in which the body’s stem cells begin to regenerate the stomach tissue with intestinal cells instead of normal stomach cells, and ultimately raising the risk of developing gastric cancer. Developing intestinal metaplasia can be the point of no return for stomach cancer because at that stage, the stomach is no longer entirely functional,” Blanke said.
In addition to keeping the gastric environment highly acidic, the stomach must also maintain a thick layer of mucus to prevent acid from degrading the stomach lining. The recent paper shows that VacA decreases that mucosal layer, which in the long-term, makes the stomach vulnerable to damage from the stomach’s acidic contents, which may also increase the risk of developing cancer.
As for the Blanke lab, its members plan to continue the experiments detailed in the paper, extending the in vivo toxin infusions from periods of 30 days to half a year or more. Additionally, they would like to attempt using the novel toxin infusion model for other pathogens as well.
“This experimental approach introduces the toxin by itself in a meaningful way, and I think it’s possible that this type of system can be especially useful for studying the role of secreted toxins during chronic infections. Right now, there’s just no straightforward way to look specifically at the effects attributable to a specific toxin over the long-term. This paper may provide a useful blueprint for allowing investigators to do just that,” Blanke said.
