
New research from University of Illinois professor William Brieher has uncovered new insights on actin disassembly.
The actin cytoskeleton is the primary determinant of cell shape and cell motility. Actin filament disassembly determines the rate at which cells can dismantle their actin cytoskeleton and rebuild it. However the mechanism of actin disassembly is still under debate, according to Brieher, a professor of cell and developmental biology.
Using microscopy, researchers Vivian Tang, Ambika Nadkarni, and Brieher have shown that actin filaments can rapidly disintegrate all along their length in a process referred to as a burst. This catastrophic event provides a mechanism for understanding how fast-moving cells like white blood cells or metastatic cancer cells manage to continuously rebuild their actin cytoskeletons by dissolving old filaments and recycling the components for another round of assembly.
The results of their research were recently published in the Journal of Biological Chemistry.
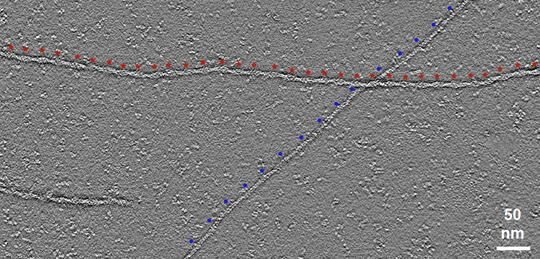
The article, “Catastrophic actin filament bursting by cofilin, Aip1, and coronin,” was selected to appear in a special virtual issue focusing on the eukaryotic cytoskeleton. Assembled by Enrique M. De La Cruz, the collection of articles explores the polymers and motor proteins that determine the function of the eukaryotic cytoskeleton in cell migration, cardiac health and neurodegeneration.
The Brieher lab in the Department of Cell and Developmental Biology is interested in understanding how cells organize an actin cytoskeleton. Projects have focused on how actin filaments are assembled at cell-cell adhesive junctions, how actin filaments disassemble, and how signals trigger actin assembly. Investigators use a combination of biochemical, biophysical, and cell biological techniques to address these questions which are fundamental for understanding cell shape, cell motility, and morphogenesis. Some of the lab’s research has provided insight into the molecular basis of inherited kidney disease.
