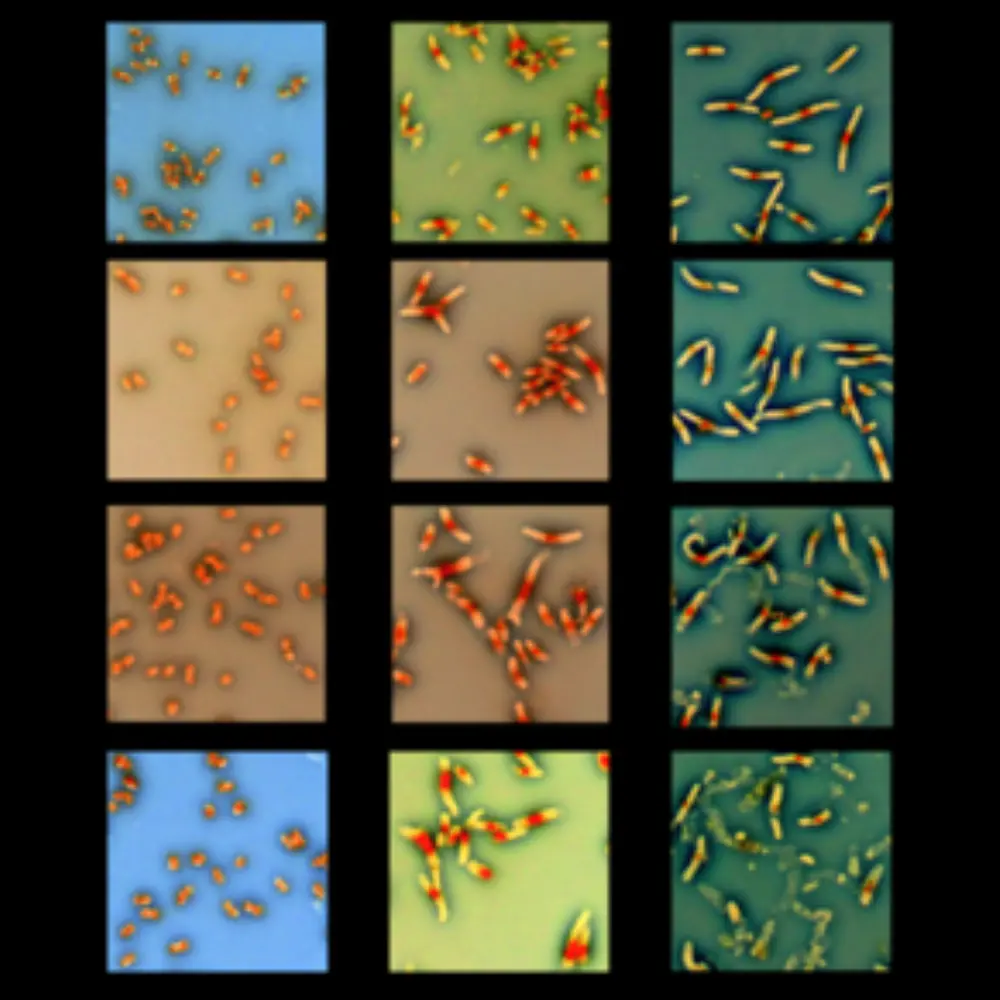
Photo caption (see right): An inverted image of DAPI-stained thyA mutant E. coli with additional defects in the enterobacterial common antigen biosynthesis under thymine starvation started by removal of thymidine from their growth medium. Left column: cells still grown in the presence of thymidine. Central column: one hour without thymidine. Right column: three hours without thymidine. Cells in the bottom row undergo massive lysis.
Andrei Kuzminov, professor of microbiology at the University of Illinois, leads research into the mystery of thymineless death, where mutant cells that cannot synthesize the essential molecule thymine will die unless the growth environment has thymine readily available. With graduate student Pritha Rao, Kuzminov recently explored the “resistance” period of thymineless death in E. coli bacteria and discovered a thymine storage mechanism in these cells, allowing them to temporarily survive without an environmental source. Their results, published in PNAS, have implications for improving the efficacy of antibiotic treatments.
Thymineless death is an area that, despite being studied for over 65 years, still puzzles scientists because of its complex mechanisms and characteristics that are different from other cell auxotrophies (metabolic requirements). Normally, when auxotrophic cells are deprived of a required supplement, they aren’t able to grow, but they don’t die off, either.
However, with thymine auxotrophs, such as the thyA E. coli mutant used by Rao and Kuzminov, the cells go through a resistance period, followed by a period of rapid exponential death. Even more strange is that during the resistance period, although cells are deprived of one of the necessary building blocks for DNA (dTTP, built around thymine), cellular DNA replication persists, replicating about half of the bacterial chromosome before the resistance period ends. This prompted researchers to ask, “Where does the thymine for this DNA replication come from?”

“We are trying to understand the mechanism of thymine-starvation-induced death, AKA thymineless death,” Rao said. “While we were investigating this phenomenon of why cells die in the absence of thymine, our research moved into a direction where we were pressed to search for an intracellular source of thymine. There were some phenotypes that could only be explained if there was residual thymine in the cell, while the cells were being starved.”
The team suspected that DNA damage was occurring after they observed DNA replication despite no environmental sources of thymine. They also observed in the microscope that thyA mutant cells extend into long filaments when deprived of thymine.
“We know this filamentation is seen when there is DNA damage in the cell. We know that thymine is a building block for DNA, so in the absence of thymine, it’s very natural to draw the conclusion that DNA must be getting damaged,” Rao said. The team’s earlier research confirmed this by showing a hotspot for DNA damage in these starved cells at the origin of replication.
Rao and Kuzminov previously tried to identify the intracellular thymine pool among high molecular weight (HMW) sources, like chromosomal DNA or stable RNA, but their experiments showed that the amount of thymine used in the resistance phase DNA synthesis could not have come from these sources. They instead turned to examine low molecular weight (LMW) sources. To identify these LMW sources, Rao and Kuzminov fed bacteria radio-labeled thymine.
“Once you feed E. coli radioactive thymine, most of it gets incorporated into the DNA, but some thymine might also become part of the intracellular LMW pool that we were looking for,” Rao said.
After separating cellular contents into LMW and HMW fractions, the researchers saw that a surprising 60% of the radioactive signal was coming from the LMW fraction, which was way higher than the expected 3%. This signal decreases to 20% during thymine starvation. Based on these observations, Kuzminov and Rao concluded that the LMW fraction was the main source of thymine used in DNA synthesis during the resistance phase of thymine starvation.
After conducting tests with various mutants defective in exopolysaccahride capsule synthesis, the team concluded that thymine-sugar precursors of these polysaccahrides form the bulk of LMW thymine. Bacterial cells use exopolysaccahrides to strengthen cell walls and for sensing the surrounding environment and contacting other cells.
“These polysacchride molecules on E. coli surface are not unlike fur on furry animals. They’re pretty long, too,” Kuzminov said.
The team also confirmed the specific roles each of the enzymes had in the production and utilization of this LMW thymine source. One of the most notable effects of inhibiting these enzymes was seen with the rffC defect.
“The thyA rffC double mutants have no resistance phase during thymine starvation and die much deeper than thyA single mutants. They experience what we call ‘hyper-thymineless death’,” Rao said. “This is one of the conditions that we have identified that has the most severe loss in viability during thymine starvation.”
The team was inspired by the steep cell death seen in rffC mutants to see if these effects could be used to boost the effects of antibacterial drugs. They tested this using trimethoprim. This drug is meant to induce thymineless death in the targeted bacterial population, but instead mostly prevents bacterial growth. However, as Rao and Kuzminov expected, the rffC mutants were dramatically killed by the same trimethoprim treatment.
As such, the team believes combination drug treatments that both inactivate the exopolysaccahride synthesis enzymes and deliver trimethoprim could be more effective than the antibacterial drug alone. This same theory could also have implications for administering thymineless death-causing drugs that treat cancer.
Kuzminov lab plans to keep unraveling the mystery of thymine starvation.
“We have known about thymineless death for 65 years, but we still do not know what it is, — although we are getting a clearer understanding of what it is not,” said Kuzminov. “This means that thymineless death mechanism must be a very complex, difficult-to-solve phenomenon. Pritha was brave to wade in and fearlessly experiment.”
