Temporal lobe epilepsy (TLE) is the most common form of epilepsy in adults, and patients often experience reproductive endocrine comorbidities, such as polycystic ovary syndrome and menstrual cycle disruption. In a new study from the University of Illinois, researchers found increased GABA transmission to neurons that regulate reproduction in female mice with epilepsy and disrupted estrous cycles. The study further helps explain links between epilepsy and reproductive comorbidities.
Anyone can develop epilepsy, a central nervous system disorder, and the origins of temporal lobe seizures are often unknown. Patients might develop temporal lobe seizures after brain injuries or years after having high fevers as children, but in many cases the cause is unclear, according to principal investigator Catherine Christian-Hinman, a professor of molecular and integrative physiology. In the Christian-Hinman laboratory, scientists have been exploring the relationship between epilepsy and reproductive endocrine comorbidities.
“In the late '80s, physicians found that patients with epilepsy had an elevated rate of developing reproductive comorbidities, such as altered semen motility in males and disrupted menstrual cycles in females,“ said Robbie Ingram, the first author of the study and a Neuroscience PhD candidate. Specifically, physicians found that there was a disrupted releasing pattern of luteinizing hormone (LH) which is important for production of key sex hormones. In a previous study from the lab, the firing rate of gonadotropin-releasing hormone (GnRH) neurons, which control LH release, was found to be disrupted in a mouse model of epilepsy.
“Starting this study, I wanted to look at the synaptic transmission to GnRH neurons and figure out why they are firing in the manner of what we are not expecting,” noted Ingram.
In this study, the Christian-Hinman lab used a commonly used technique to induce a specific model of epilepsy by injecting a small amount of kainic acid on one side of the hippocampus. This technique will pharmacologically induce seizures that later develop into epilepsy in animals and allows researchers to see changes within mice with epilepsy, according to Christian-Hinman.
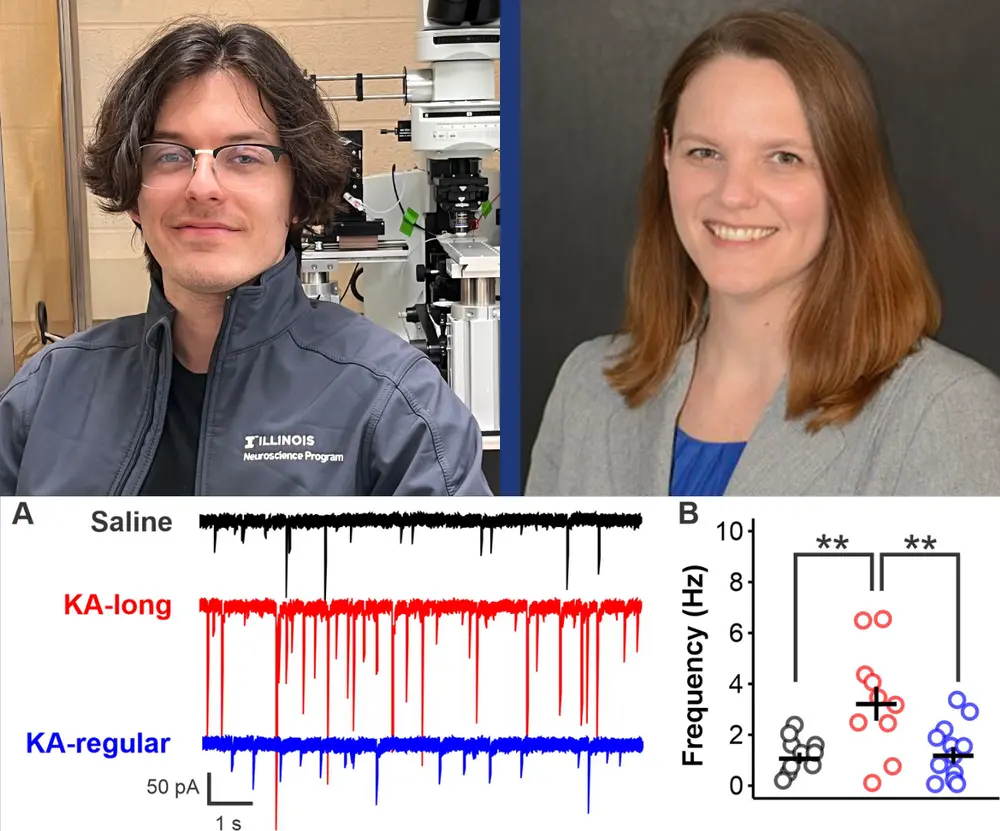
In the current study, researchers observed an increase in transmission of the neurotransmitter GABA to GnRH neurons in mice that developed epilepsy after kainic acid injection. The female mice that showed an increase in GABA transmission also exhibited reproductive comorbidities such as lengthened and disrupted estrous cycle compared with control animals and females with epilepsy that maintained regular cycles. However, the researchers did not see the increase in GABA transmission in kainic acid-injected males, Ingram said.
“From this study, we can derive a model where GABA plays a role in driving the change in firing in GnRH neurons that we have seen in our past studies,” Christian-Hinman said.
Furthermore, Ingram and Christian-Hinman found that blocking receptors for another major transmitter, glutamate, blocked the change in GABA transmission. This is important because they not only see what happens at GnRH neurons but also one step up of the circuit as well. “This surprising detail has informed our proposed working model where altered glutamate transmission to the upstream GABA neurons can increase GABA transmission in GnRH neurons, and we think that a group of GABA neurons in a region called the AVPV might be the source,” Ingram explained.
Looking ahead, Ingram would like to use this proposed working model to see how AVPV neurons drive the transmission to GnRH neurons and cause alteration in firing.
“Some treatments are not suggested for people with epilepsy who have these comorbidities because they are thought to complicate the picture, so they are not good candidates for certain drugs. Therefore, the more that we can understand about solving the problem of comorbidities, it will also help us solve the problem of epilepsy itself by expanding potential treatment options,” Christian-Hinman said.
The article “Increased GABA transmission to GnRH neurons after intrahippocampal kainic acid injection in mice is sex-specific and associated with estrous cycle disruption” is available online.
This work was supported by National Institutes Health/National Institute of Neurological Disorders and Stroke and CURE Epilepsy Research Continuity Fund Grant.
