
Contact Information
Research Interests
Research Topics
Drug Discovery, Endocrinology, Metabolic Regulation, Neurobiology, Optogenetics, Reproductive Biology
Disease Research Interests
Metabolic Disorders/Diabetes, Neurological and Behavioral Disorders, Reproductive Diseases, Infertility, and Menopause
Research Description
Neural circuitry regulating feeding and emotion
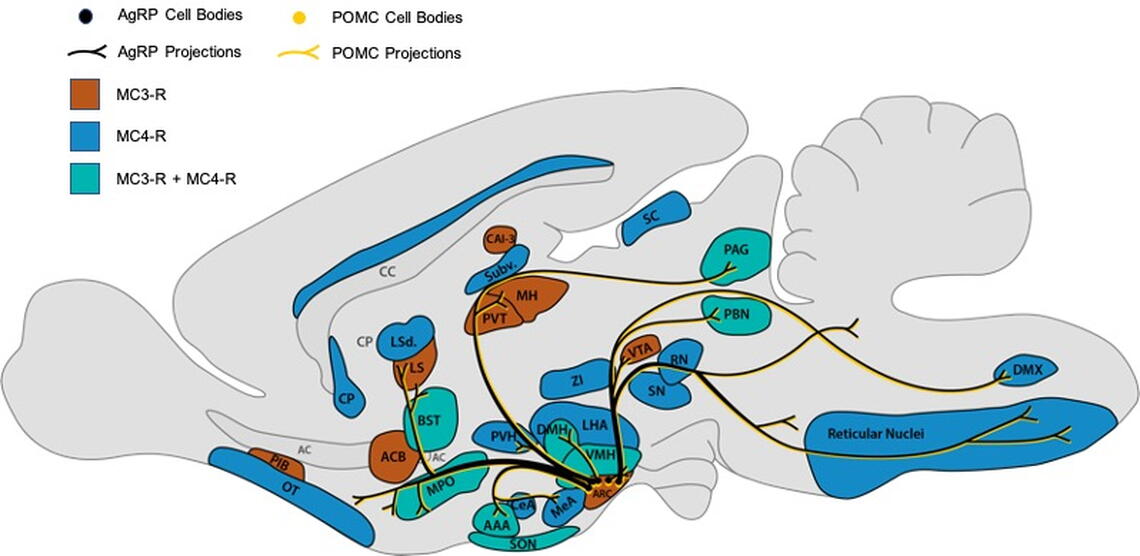
Feeding behavior is largely controlled by conserved neural circuitry located in the hypothalamus. These neural circuits are interconnected with brain circuits that regulate diverse physiological and emotional processes, and dysfunction in this circuitry is likely at the core of many metabolic and psychiatric disorders, such as anorexia nervosa and obesity. To better understand the neural circuitry controlling feeding, and how this circuitry is altered in pathological conditions, we focus our studies on the central melanocortin system. The melanocortin system is well conserved across species and is critically involved in communicating metabolic state with diverse neural circuitry controlling emotion, behavior, and physiology (Fig. 1). The melanocortin system is composed of “first order” pro-opiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons located in the hypothalamic arcuate nucleus (Figs. 1 and 2). These neurons directly sense changes in stored energy via the fat secreted factor leptin, and communicate this information to diverse secondary sites throughout the brain (Figs. 1 and 2). POMC and AgRP neurons produce the endogenous melanocortin receptor agonist alpha-melanocyte stimulating hormone (aMSH) or the endogenous melanocortin receptor antagonist/inverse agonist AgRP, which together act on central melanocortin receptors (MC3R and MC4R) distributed throughout the brain (Figs. 1 and 2)
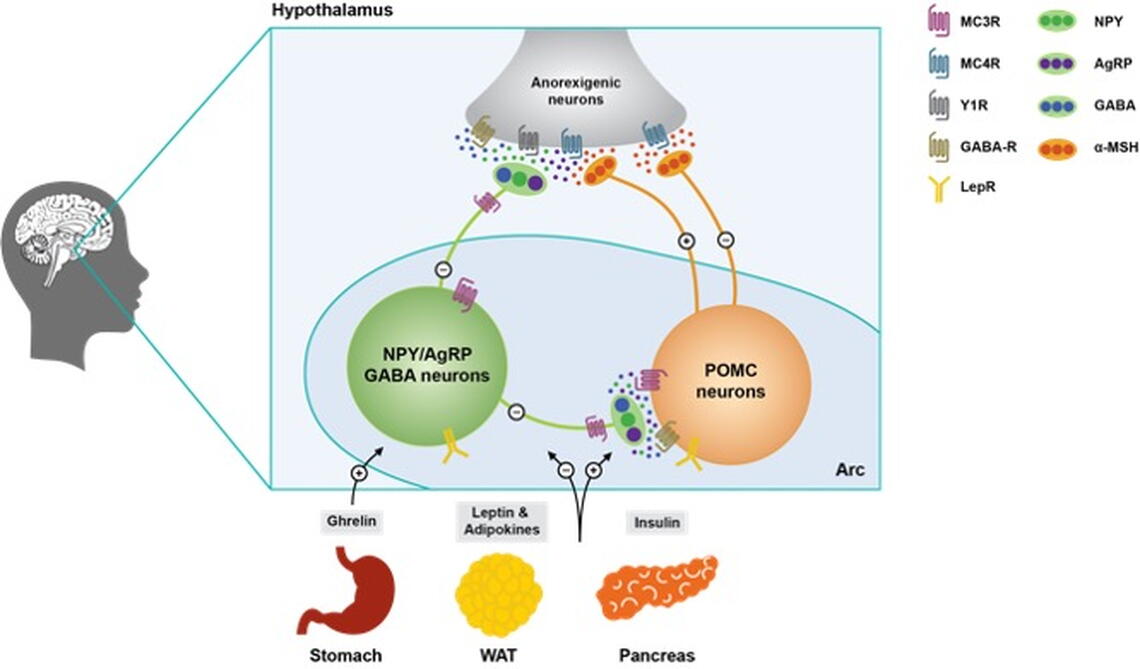
Importantly, mutations in the central melanocortin pathway produce profound obesity across diverse species, and mutations in the MC4R are the most common cause of severe monogenetic obesity in humans, occurring as frequently as 1:500 individuals. Thus, this circuitry provides a well validated and physiologically relevant system for studying how metabolic state influences motivation and emotion, and for determining how these interactions are disturbed in diseases characterized by pathological feeding behavior. Current studies in the lab are focused on characterizing the response of MC3R and MC4R expressing neurons in physiological and pathological forms of feeding, and determining how POMC and AgRP neurons relay information to secondary melanocortin receptor sites throughout the brain (Fig. 1). To accomplish this goal the lab employs a multidisciplinary approach including in vivo calcium imaging (Inscopix miniscope imaging), optogenetics, chemogenetics, mouse molecular genetics, neuropharmacology, light sheet imaging of neuronal circuitry, and single cell/single neuron RNA sequencing. We hope that these studies will provide new approaches for treating human diseases at the intersection of energy homeostasis and emotion, such as anorexia nervosa.
Education
B.A. 2012 University of Rochester
Ph.D. 2017 SUNY Upstate Medical University
Postdoc. 2017-2021 University of Michigan
Additional Campus Affiliations
External Links
Highlighted Publications
Representative Publications
1. Sweeney P, Bedenbaugh M, Pan P, Fowler K, Williams S, Gimenez L, Downing G, Joy S, Mapp A, Simerly R.B, Cone R.D. (2021) Melanocortin 3 receptor is a pharmacological target for the regulation of anorexia. Science Translational Medicine.
2. Zhang J, Chen D, Sweeney P, Yang Y. (2020) An excitatory hypothalamus to paraventricular thalamus (PVT) circuit that suppresses food intake. Nature Communications.
3. Sweeney P, Chen C, Rajapakse I, Cone R.D. (2021) Network dynamics of hypothalamic feeding neurons. Proc. Natl. Acad. Sci. USA
4. Ghamari-Langroudi M, Cakir I, Lippert RN, Sweeney P, Litt MJ, Ellacott KLJ, Cone RD. (2018) Regulation of energy rheostasis by the melanocortin-3 receptor. Sci Adv. Aug;4(8).
5. Sweeney P., Li. C, Yang. Y (2017). Appetite suppressive role of medial septal area glutamatergic neurons. Proc. Natl. Acad. Sci. USA. 114(52):13816-13821 (Featured in Nature)
6. Sweeney P., O’Hara. K, Xu. Z, and Yang. Y (2017). HFD-induced energy states-dependent bidirectional control of anxiety levels in mice. Int J Obes (Nature press; London). 41(8):1237-1245.
7. Sweeney P. & Yang. Y (2017). Neural circuit mechanisms underlying emotional regulation of homeostatic feeding. Trends in Endocrinology & Metabolism. 6(28), 437-448.
8. Sweeney P, Qi. Y, Xu Z, and Yang. Y (2016). Activation of hypothalamic astrocytes suppresses feeding without altering emotional states. Glia. 64 (12), 2263-2273.
9. Sweeney P and Yang. Y (2016). An inhibitory septum to lateral hypothalamus circuit that suppresses feeding. Journal of Neuroscience. 36 (44): 11185-11195. (Featured article)
10. Sweeney P, and Yang. Y (2015). An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nature Communications. 6: 10188. (Featured by Faculty of 1000)
Recent Publications
Possa-Paranhos, I. C., Butts, J., Pyszka, E., Nelson, C., Congdon, S., Cho, D., & Sweeney, P. (2025). Medial hypothalamic MC3R signalling regulates energy rheostasis in adult mice. Journal of Physiology, 603(2), 379-410. https://doi.org/10.1113/JP286699
Butts, J., & Sweeney, P. (2024). A neural pathway integrating stress and feeding. Neuropsychopharmacology, 49(9), 1357-1358. https://doi.org/10.1038/s41386-024-01890-7
Catalbas, K., Pattnaik, T., Congdon, S., Nelson, C., Villano, L. C., & Sweeney, P. (2024). Hypothalamic AgRP neurons regulate the hyperphagia of lactation. Molecular Metabolism, 86, Article 101975. https://doi.org/10.1016/j.molmet.2024.101975
Dahir, N. S., Gui, Y., Wu, Y., Sweeney, P. R., Rouault, A. A. J., Williams, S. Y., Gimenez, L. E., Sawyer, T. K., Joy, S. T., Mapp, A. K., & Cone, R. D. (2024). Subthreshold activation of the melanocortin system causes generalized sensitization to anorectic agents in mice. Journal of Clinical Investigation, 134(14), Article e178250. https://doi.org/10.1172/JCI178250
Cho, D., O’Berry, K., Possa-Paranhos, I. C., Butts, J., Palanikumar, N., & Sweeney, P. (2023). Paraventricular Thalamic MC3R Circuits Link Energy Homeostasis with Anxiety-Related Behavior. Journal of Neuroscience, 43(36), 6280-6296. https://doi.org/10.1523/JNEUROSCI.0704-23.2023




