November 20, 2013
Image
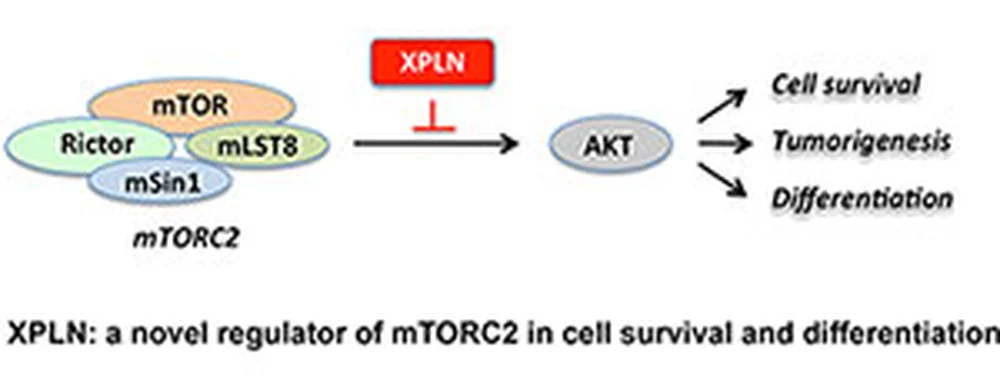
The protein kinase mammalian target of rapamycin (mTOR) is a master regulator of cell growth, survival, differentiation, and metabolism.
The protein kinase mammalian target of rapamycin (mTOR) is a master regulator of cell growth, survival, differentiation, and metabolism. Two biochemically unique protein complexes of mTOR – mTORC1 and mTORC2 – have distinct kinase activities and assemble signaling pathways that differentially regulate those cellular and developmental processes. As hyper-activation of mTOR signaling underlies many human diseases including diabetes and cancer, Nature has most likely designed intrinsic breaks for these pathways. In this study led by Nidhi Khanna, a graduate student in the Chen laboratory, one such control mechanism has been identified for mTORC2. The guanine nucleotide exchange factor (GEF) for Rho small GTPases, XPLN, was first found by the researchers to interact with mTOR in a yeast two-hybrid screen. Experiments in mammalian cells subsequently revealed that XPLN specifically interacts with mTORC2, not mTORC1. XPLN inhibits mTORC2 kinase activity in vitro and signaling in cells. The inhibitory function of XPLN is specific for one of the targets of mTORC2 – the protein kinase Akt. In line with the well-established functions of Akt, XPLN negatively regulates cell survival and skeletal muscle differentiation through its inhibitory effect on mTORC2 and Akt. Interestingly, this novel function of XPLN does not require its GEF activity, but is instead dependent on its N-terminal region, most likely via direct physical interaction with mTORC2. As hyper-active Akt signaling is oncogenic and found in many human cancers, the discovery of the first mTORC2-specific endogenous inhibitor raises the possibility of tumor-suppressing function for XPLN, an intriguing question for future investigations.Story Source(s)