August 25, 2014
Image
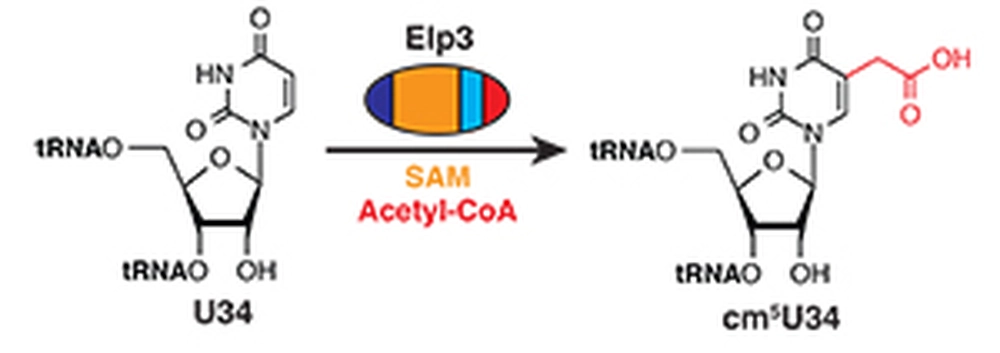
In the latest issue of Nature Chemical Biology, biochemistry graduate student Kiruthika Selvadurai and associate professor Raven Huang reported their findings that the chemistry of eukaryotic tRNA modification at the “wobble” position is performed entirely by a single subunit of the six-subunit Elongator complex, and involves formation of a highly unusual radical intermediate on acetyl-CoA.
Approximately a quarter of cytoplasmic tRNAs in eukaryotic organisms contain a modified uridine at the wobble position, which plays a crucial role in maintaining the efficiency and fidelity of protein translation. The lack of this modification severely affects translation of several important proteins whose genes use biased codons that require modified tRNAs. Genetic studies indicated that the eukaryotic Elongator complex, which consists of six subunits of Elongator protein (Elp1-Elp6), performs the central step of the modification, but the mechanism was unknown. In humans, defects in the Elongator complex have been linked to several neurological diseases such as familial dysautonomia (FD), rolandic epilepsy (RE), and amyotrophic lateral sclerosis (ALS). To provide insight into the mechanism of the modification reaction carried out by the Elongator complex, Huang and coworkers first performed bioinformatic analyses and found that only Elp3 (not Elp1-2 and Elp4-6) is present in archaea, which lacks the genes responsible for a distinct tRNA modification found in bacteria. This knowledge, along with the fact that Elp3 possesses a radical SAM and a HAT domain, led the researchers to hypothesize that tRNA wobble uridine modification in archaea is similar to the one found in eukaryota, and only Elp3 is required for catalysis. This hypothesis was subsequently confirmed by their in vitro reconstitution experiment using a recombinant archaeal Elp3 protein. Huang commented that, among several interesting mechanistic details of the Elp3-catalyzed reaction revealed by additional chromatographic and spectrometric experiments, generating a radical on the methyl group of acetyl-CoA is particularly significant. Acetyl-CoA can be regarded as “the carbon currency” of living organisms, as the overwhelming majority of carbon metabolism goes through it. To their knowledge, this is the first example of a radical reaction occurring on the methyl group of acetyl-CoA, providing potentially new tools for biosynthesis/modification of natural products and macromolecules in living organisms that require formation of a carbon-carbon bond.Story Source(s)