
Through the ages, fertility has played a central role in civilizations. Its cultural, socioeconomic, demographic, religious and global implications were and still are incalculable. A Wikipedia search for “fertility gods,” for example, yields a list of 35 cultures with one, and often multiple, fertility deities. Cultural associations of fertility with happiness and productivity are woven into the
very fabric of societies. In today’s world, societies are concerned about the role that their fertility rate may play in global economic and political affairs.
Pioneering research in the Department of Molecular and Integrative Physiology (MIP) at the University of Illinois at Urbana-Champaign currently focuses on factors that influence uterine receptivity and successful implantation, the ingredients of a successful pregnancy. Placental abnormalities are being studied in addition to endometriosis, a painful disease affecting up to 15% of reproductive age women. A novel approach to endometriosis has led to an exciting break- through—two potential agents to treat it!
The laboratories of Milan Bagchi, Professor and Head of the Department of MIP and of Indrani Bagchi, Professor of Comparative Biosciences, have collaborated to define the molecular pathways governing implantation.Their work has increased our understanding of the causes of infertility, endometriosis and even endometrial (uterine) cancer.
In recent years, ART (assisted reproductive technology) has benefited some childless couples, but the success of ART is dependent upon a receptive uterus to which the fertilized embryo can attach and develop. Using mouse models and cultures of human endometrial cells, the Bagchi laboratories have demonstrated how the intracellular estrogen and progesterone receptors bind to specific DNA sequences in the genome, thereby activating or repressing the expression of specific genes. These receptors regulate transcription of target genes to produce messenger RNA, which are subsequently translated into proteins that mediate specific cellular functions (Figure 1).
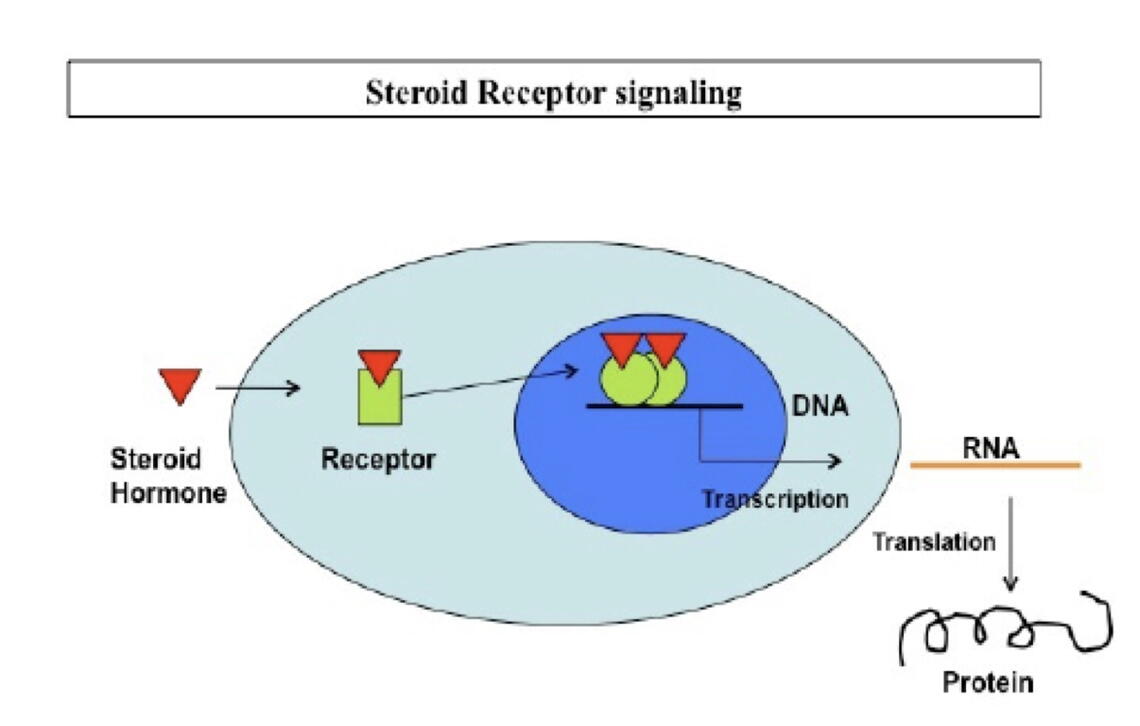
Estrogen (E) and progesterone (P) both play critical complementary roles in preparing the uterus for successful implantation of the embryo. Estrogen promotes growth of the uterine lining composed of epithelial cells just before ovulation, and progesterone levels then increase after ovulation to remodel the uterine lining, glands and deeper stromal compartment, a process known as decidualization (Figure 2). A sequence of precisely timed, complex molecular pathways involved in the communication between the epithelial and stromal uterine cells, driven by E and P, are essential for implantation (Pawar et al., Mol Endocrinol. 28:1408-22, 2014).
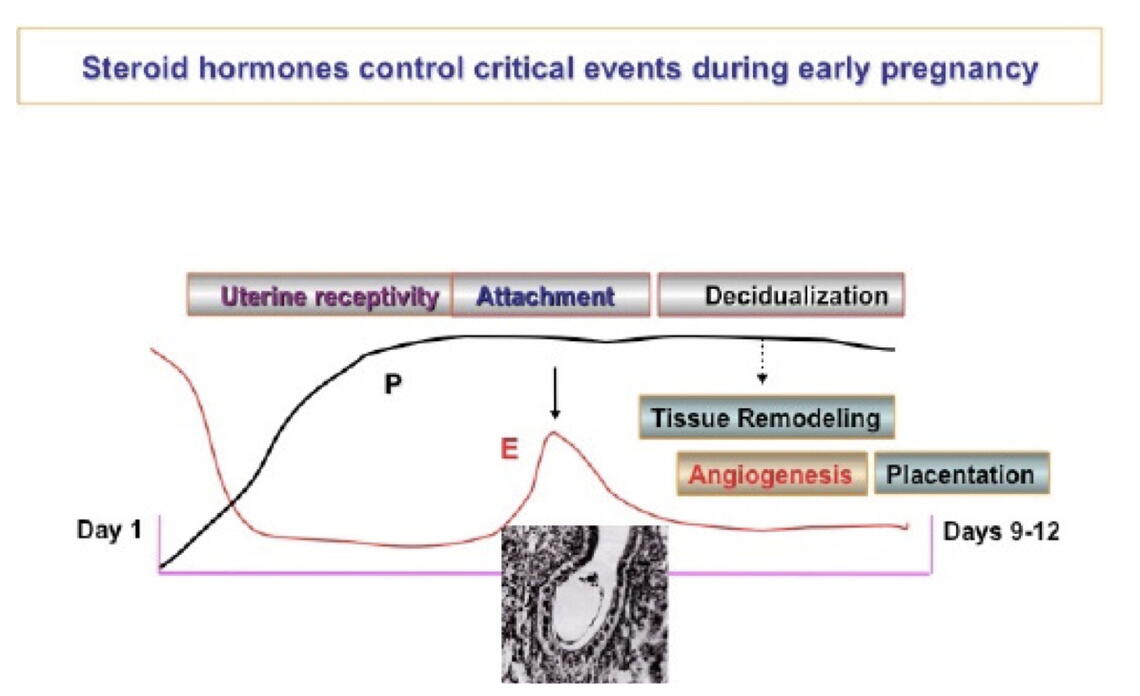
The fact that P inhibits proliferation of the epithelial cells has been known for many years, but the Bagchi laboratories have discovered that a gene, HAND2, is a major driver of this action, acting as a “brake” on epithelial cell proliferation by inhibiting the production of fibroblast growth factors in the uterine stroma (Li et al., Science 331: 912-916, 2011). By deleting HAND2 in mice, they have shown that the epithelial cells proliferate in an uncontrolled manner, creating uterine tissue that is unreceptive to an embryo and resulting in implantation failure. Deletion of the HAND2 gene has also been shown to “down-regulate” a tumor suppressor gene, Pten, an event that can lead to the development of uterine carcinoma.
Epigenetics lies at the heart of this research. The human genome consists of approximately 30,000 genes, each containing information incorporated in nucleic acid base pairs (DNA) arranged in a specific sequence. However, various environmental influences (e.g. exposure to endocrine disrupting agents) can activate or suppress a given gene without changing the sequence of its DNA. Interestingly, the epigenetic alteration of the gene HAND2, resulting from a chemical modification, known as methylation, at specific sites in the gene, has the effect of suppressing its action (Jones et al., PLoS Medicine 10: e1001551, 2013). The “braking” capability of HAND2 is thereby compromised, which then drives epithelial cell proliferation and pre- malignant cellular changes, precursors to endometrial (uterine) cancer. The HAND2 research results may be helpful in the development of early detection methods for uterine cancer and new treatment options as well.
Endometriosis affects up to 15% of reproductive-age women (perhaps higher than 15% due to failure to diagnose this condition) and is characterized by endometrium-like tissue deposits located in sites outside the uterus such as the peritoneal lining in the pelvis and the ovaries. These deposits, which actually show molecular characteristics that are distinct from normal endometrium, can be painful and also cause infertility due to scarring of the fallopian tubes and ovaries. It has been known for many years that estrogen (E) is a driver of endometriosis. E binds to nuclear proteins known as alpha and beta estrogen receptors which, in turn, act as signaling proteins in complex pathways to regulate the activity of numerous genes. As a result, an array of inflammatory proteins is produced, and the development of nerves and blood vessels in the abnormal deposits is promoted, resulting in pain, a cardinal symptom of endometriosis.
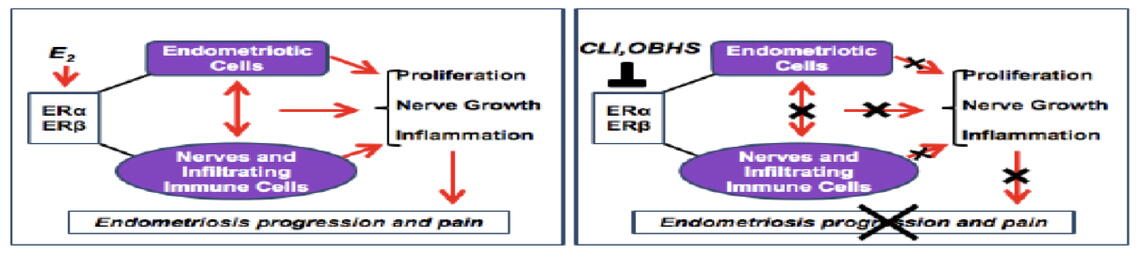
Therapies for endometriosis have traditionally been directed towards reducing blood levels
of E, but side effects resulted, and the inflammatory process was not dampened. Dr. Benita Katzenellenbogen (Department of MIP), who has intensively studied the estrogen and progesterone receptors for many years, and Dr. John Katzenellenbogen (Department of Chemistry), a renowned chemist, have developed two different drug compounds, chloroindazole (CLI) and oxabicyclo-heptene sulfonate (OBHS), which bind strongly to the beta and alpha estrogen receptors respectively (Zhao et al., Sci Transl Med. 7:271ra9, 2015). These compounds, which don’t compromise fertility, have a dual action: (1) they block the establishment of endometriotic lesions and reduce the size of existing lesions; and (2) they exhibit strong anti-inflammatory action by blocking macrophage-induced production of inflammatory cytokines.
The role of inflammation, highlighted by the endometriosis research projects described above, is gaining increasing attention in a wide spectrum of human disease. Of interest is the fact that CLI has also been shown to be a successful treatment for multiple sclerosis (MS) in mice. CLI and OBHS show promise as potential treatments for endometriosis, but because of the ability of these compounds to suppress inflammation in a hyperestrogenic environment, they may also prove useful, after appropriate clinical testing, in the treatment of certain breast cancers, some lung disorders, liver fibrosis and cardiovascular and metabolic effects related to obesity.
An intriguing area of research in the laboratory of MIP Professor Derek Wildman centers on the placenta. This research focusing on disorders of placentation can enable understanding of obstetrical syndromes such as pre-term birth and pre-eclampsia. Pre-eclampsia is a common cause of a failed pregnancy. The wide variation in placental shape, size, type of blood flow and type of fetal-maternal interfaces within species and across species is large, and this makes it difficult to find animal models to study human diseases of placental dysfunction. Nevertheless, Dr.Wildman has been able to show, by genetic analysis, that our closest ancestors (chimpanzees and gorillas) adaptively evolved towards a placenta resembling modern humans.
A fascinating area of placental research centers on the ability of the placenta to adapt to environmental challenges such as hypoxia and poor nutrition. In a recent scientific paper, Dr. Wildman describes how native populations in high altitudes (hypoxic) in the Andes and in Tibet have adapted to these conditions by selecting for genes that enhance placental oxygen exchange between mother and fetus (Gundling and Wildman, Philos Trans R Soc Lond B Biol Sci. 370(1663):20140072, 2015). Recent migrants to high altitude settings deliver low birthweight infants, since they haven’t yet adapted to the hypoxic environment, while native women deliver normal birthweight infants. Interestingly, the Andean and Tibetan populations, living great distances apart from one another, have separately evolved these adaptive genomic responses (convergent evolution).
Another example of placental plasticity or adaptability involved Dutch women during a famine in World War II. Epigenetic studies utilizing blood samples from individuals who were conceived during, before, and after the famine revealed differential expression (activation) of the placental genes in these three populations affecting nutrient exchange and fetal growth.
Women’s reproductive health plays a critical role in society, and research in this area, while severely underfunded, expands our understanding not just of reproductive health issues but also of broader subjects such as epigenetics and evolution, integral to all biological systems. This research is already guiding us towards early detection and treatment methods for endometriosis and uterine cancer, and it has underlined the importance of inflammation as a common denominator in a whole spectrum of diseases ranging from MS to obesity.

