New findings suggest that novel down-regulation of GIRK channels by caspase-3 may contribute to NMDAR-dependent hippocampal atrophy following chronic epileptic seizures
Epilepsy is a chronic brain disorder characterized by recurrent epileptic seizures indicative of excessive neuronal activity. Temporal lobe epilepsy (TLE) is the most common epilepsy in adults. TLE is associated with drug-resistant seizures, cognitive decline, and progressive neuronal death in the hippocampus, a brain region important for learning and memory. Such hippocampal sclerosis has been associated with excessive activation of N-methyl-D-aspartate receptors (NMDARs) and stimulation of cysteinyl aspartate-specific protease (caspase) family proteins involved in programmed cell death. However, the identity of caspase substrates that contribute to seizure-induced hippocampal atrophy remains largely unknown.
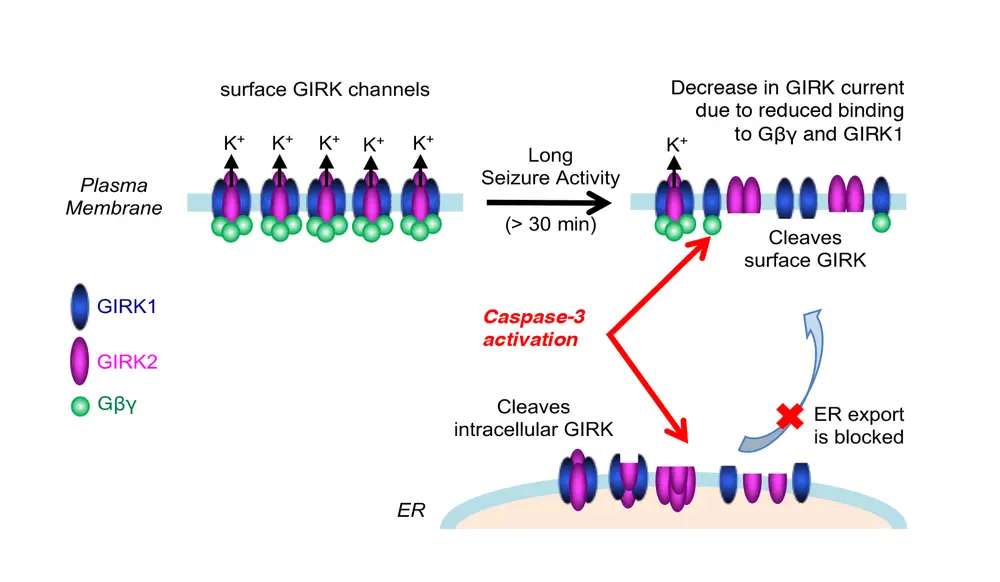
In a study published in Scientific Reports, co-lead by Brian Baculis (a graduate student) Amanda Weiss (a research technician), and Weilun Pang (an MCB undergraduate student), Dr. Chung’s laboratory discovered that prolonged high-frequency epileptiform discharges in hippocampal neurons in culture and in mice lead to caspase-dependent cleavage of GIRK1 and GIRK2, the major subunits of neuronal G protein-activated inwardly rectifying potassium (GIRK) channels that mediate membrane hyperpolarization and synaptic inhibition in the brain. Such cleavage severely disrupts channel assembly, channel activation by G proteins, and trafficking to the plasma membrane. These findings suggest that this novel down-regulation of GIRK channels by caspase-3 may contribute to NMDAR-dependent hippocampal atrophy following chronic epileptic seizures.

