Danielle Campbell, a microbiology PhD graduate of the Whitaker Lab, recently studied the interaction of the active prophage, Bacteroides phage BV01, in its tractable host strain, B. vulgatus ATCC 8482. Bacteroides, known to degrade complex carbohydrates and interact with host immune cells, are one of the most common bacterial types in the human gut. The gut phageome is linked to diseases including inflammatory bowel disease, malnutrition, HIV/AIDS, and colorectal cancer, among others. By altering the activity of their bacterial hosts, gut-associated phages contribute to these effects in human health, making it an exciting field of research.
Humans host many commensals that are harmless, and sometimes beneficial, to our health. While the effect of phage-host interactions on virulence is well understood, phage-host interactions themselves are relatively underexplored. Campbell’s paper, “Infection with Bacteroides Phage BV01 Alters the Host Transcriptome and Bile Acid Metabolism in a Common Human Gut Microbe,” was published in Cell Reports. In her study, Campbell describes how the physical integration of BV01 DNA into host bacterial DNA is disruptive, however, this is not the only form of phage-host interaction. There is great diversity in bacterial hosts and their corresponding phage interactions. New mechanisms for bacteria-phage interactions are constantly being discovered.

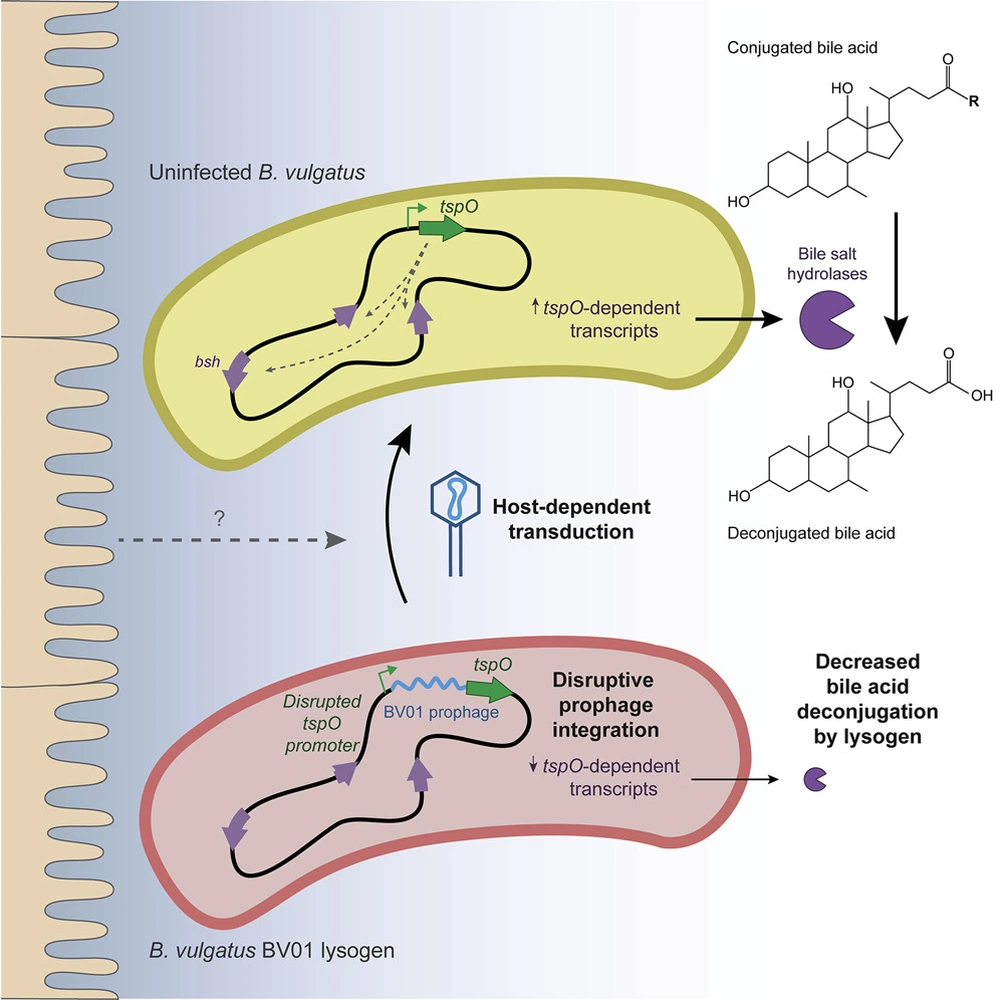
Campbell’s study revealed that BV01 broadly altered its host transcriptome through repression of the tryptophan-rich sensory protein (TspO). The phage induced disruption of the tspO promoter in vitro subsequently repressed the deconjugation of bile acids. Bile acids are important for mammalian host signaling, so repression of bile acid deconjugation has the potential to alter host phenotypes. Since this deconjugation is specifically common in gut-associated microbes, its direct benefit to the microbe is unclear. However, modification to the bile acid pool could be linked to beneficial effects on human host metabolism.
Campbell’s research differs from other microbe studies because of the utilization of bacteria, pre-infected with a virus. Many other studies initially find a virus through DNA sequencing and work backward to identify and grow a host for the phage. This process can be extremely arduous and challenging, Campbell said. Her approach fast-tracks this process and has provided a useful model for future studies investigating gut microbe phage-host interactions.
Additionally, the potential metabolic effects of BV01 could be utilized in clinical applications. Unlike probiotics, which pass through the host fairly quickly, phages will remain within their mammalian host as long as their bacterial host is present, therefore, having the potential to have more stable effects.
In the past, researchers have been unable to identify phages that can effectively infect Bacteroides, however, with their recent findings on the Bacteroides phage BV01, there is potential for the phage to be utilized as a genetic engineering tool in bacteria. Campbell and her colleagues’ discoveries on BV01, a phage that stably integrates into Bacteroides, show great promise for future gut-associated phage studies.
