
Epithelial cells, which cover our body and line our organs, have several functions which make them critical for certain bodily processes. To carry out their various physiological functions, these cells rely on apical junctions, specialized cell-cell junctions which exert a force that will carry out dynamic processes such as wound migration and morphogenesis through their interactions with actomyosin.
Researchers from the University of Illinois School of Molecular & Cellular Biology have discovered that functions of a new motorized organelle challenge the existing model of epithelial homeostasis. The project, six years in the making, was led by Vivian Tang, Research Assistant Professor in the Department of Cell & Developmental Biology, and three students who were undergraduate researchers in her lab: Timothy Morris, BS, ’19, Molecular & Cellular Biology (MCB); Eva Sue, BS, ’21, MCB; and Caleb Geniesse, BS, ’14, MCB. Their findings were published in “Synaptopodin stress fiber and contractomere at the epithelial junction” in the Journal of Cell Biology.
“The undergraduate researchers really contributed to the project, and each did so in a different way. I am impressed by their dedication and continual interests in the project even after they graduated,” Tang said.
"I came into the Tang lab with the goals of being able to perform research with a higher level of interaction with the principal investigator,” said Morris, who is now a medical student at the University of Iowa. “After my time with the lab members, I learned not only about research, but I learned life lessons and advice for my future career. Dr. Tang was always supportive of me going into medicine while being in her lab. The research I performed helped me get more research published while in medical school and learn how to read papers and create my own analysis of the data.”
For this endeavor, Morris zeroed in on molecular biology research. Caleb Geniesse, currently a PhD candidate at Stanford University, focused on the biochemistry work behind the project. Eva Sue, who is now studying bioinformatics at the University of Chicago, generated cell lines and conducted multicell analysis.
The new organelle, which they named contractomere, possesses the ability to alter the proportions of the apical junction and the cell-packing geometry of an epithelial monolayer. These novel functions alter the current dogma of junction dynamics.
For a mechanical force to be transmitted from one cell to another, a physical linkage is necessary, Tang said. However, details of these linkages are relatively unknown in the field. Scientists have established that adhesion receptors allow for the linkage between two cells. There is a direct connection between the adhesion receptors and the actomyosin cytoskeleton, where the forces are generated. When considering the role of contractomeres in these linkages, “there is a new player in town,” she said.
Her group’s discovery of the contractomere, which walks around the perimeter of a cell while simultaneously rearranging it, highlights an entirely new mechanism of organizing cell boundaries. The contractomere is also force-generating but in a very different way than what was known prior to this study.
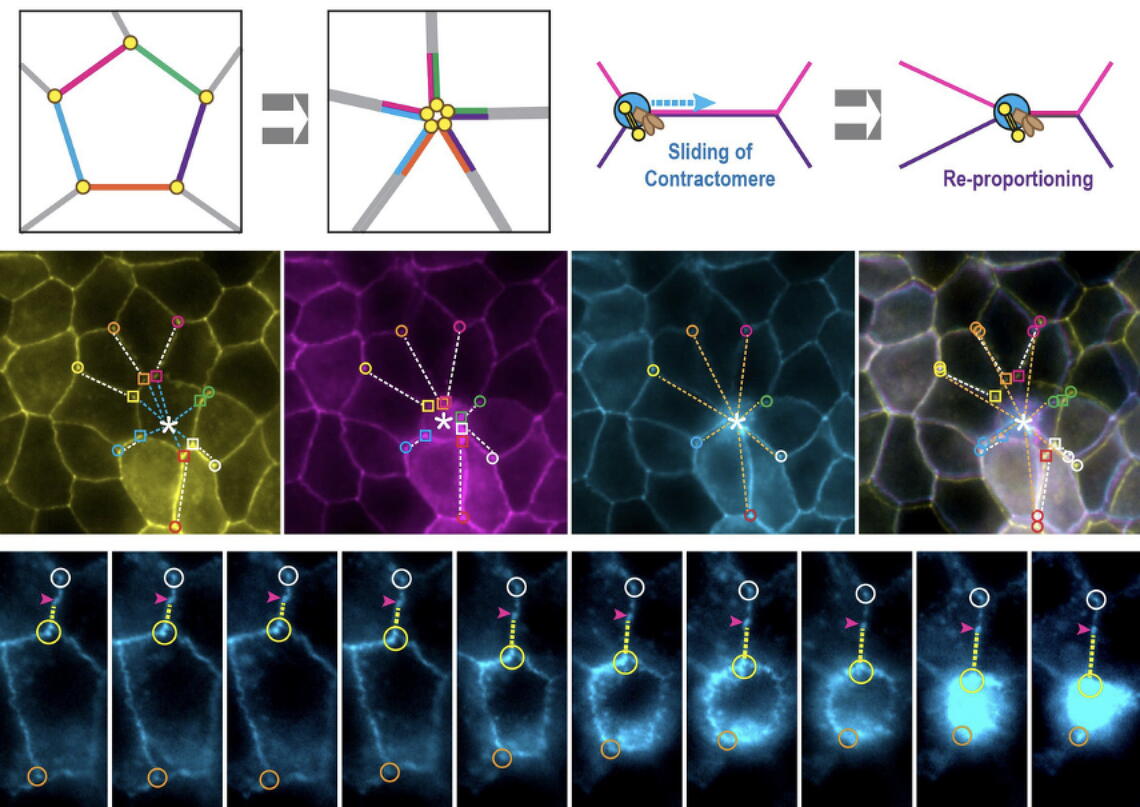
The contractomere “requires a local compressive force to propel itself. This is very different from other models that imagine the cytoplasm generating force and pulling on the adhesions,” Tang said.
Investigating the distinctive functions of this novel organelle may provide insight into the mechanisms of maintaining epithelial homeostasis. Epithelial cells have a high turnover rate. Their regeneration requires a sheath of cells to close the area where cells are extruded. Inefficient closing of this area will result in a leaky epithelium where toxins can enter. Previous scientific models rely on the membrane recycling mechanism, which is not an efficient method to maintain epithelial homeostasis.
“Prior to [our] paper, current models could not explain how the cells heal so quickly and preserve the barrier function of the epithelium. Through our model, we can explain how it closes so quickly,” Tang said.
The breakthrough functionalities of contractomeres challenge what scientists currently know about junction dynamics. Tang will be presenting the research this summer at the Gordon Research Conference, an international forum highlighting research in biological, chemical, physical, and engineering sciences. Tang and her colleagues plan to further explore the molecular components and mechanisms of the adhesion junction.
As for her former students, working in a lab provide valuable experiences for them, and in the case of Geniesse, changed his trajectory.
“I initially saw it as an opportunity to boost my resume for med school, but it opened my eyes to the world of research and inspired me to go to grad school instead.” Geniesse said. “My research experience as an undergrad not only introduced me to this new career path, but it also gave me the technical background I needed to succeed as a PhD candidate,” he said. At Stanford, he’s developing new computational methods for capturing and quantifying fluctuations in brain dynamics.
Added Sue: “The experience really challenged me to think outside of the classroom. I was able to apply things I had learned in the MCB curriculum to a real-world research context, and the experience showed me how much impact research has. Although I am not pursuing research in my current career trajectory, I have gained invaluable skills and a research mindset that still influence my work in my graduate studies.”