Although researchers have been able to achieve many advances in regenerative medicine, repairing damage in the nervous system of the human body has stubbornly eluded scientists and doctors. The nervous system and the brain present many obstacles that, until recently, have had few effective solutions. Neuronal cells are notoriously slow to multiply as humans mature. Repairing damage to these systems in the brain is also a troublesome and problematic task. These factors, combined with the challenge of getting neuronal cells to grow in specific patterns or directions after an injury, makes finding a solution at best daunting and at worst, seemingly impossible, according to researchers.
The brain has several mechanisms that render current therapeutic approaches used in other portions of the body ineffective. The blood-brain barrier prevents the transmission of many therapeutic compounds to cells beyond. The brain also has an extremely delicate and specific microenvironment for cells in different structures, so dosing a wide area with any treatment could potentially do more harm than good. Finally, an entirely new problem is presented if one wants to control the growth of one cell, or smaller still in specific dendrites or the axon of a neuron. The consequence of all these difficulties is that if someone sustains an injury and neurons are damaged, it is almost always not repairable, and treatment has undesirable effects.

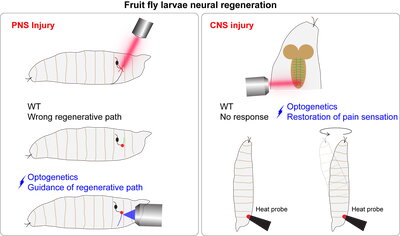
However, new research by biochemistry professor Kai Zhang and his team at the University of Illinois uses the groundbreaking field of optogenetics to show a potential new route for controlling specific neuron growth with incredible accuracy. Their recent paper “Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila,” published in eLife and featured in eLife Digest, shows that optical activation of Raf and AKT pathways can trigger highly tunable cell growth.
These traditional cell growth pathways have been previous targets of therapeutics to trigger cell growth, but previous attempts have failed due to the inability to contain the treatment to a single cell, and the highly invasive nature of implanted devices or delivered therapeutic chemicals. In this new optogenetic method, a laser beam is directed very precisely at cell axons or dendrites to incite growth, and the direction of the light can control the specific path axon growth takes.
One of the most exciting pieces of this study is that optogenetics allows for bypassing of cell ligand receptors and can directly activate the associated downstream effects in confined regions of a cell, according to Zhang. This minimizes background noise and the risk of activating other receptors within the same complex unintentionally, or triggering other undesired downstream effects caused by the same receptor.
“Very rarely do you hear about activating these pathways only in a specific part of the cell,” Zhang said. “Turning on the growth receptor pathway at different locations could totally change the cell fate.” Zhang and project collaborators, Dr. Yuanquan Song at the Children’s Hospital of Philadelphia, are excited about the potential applications of this work, and for the contribution to the young field of optogenetics. “I believe [optogenetics] has a lot of potentials to open up new opportunities,” Zhang said. “We just need to show the community, case-by-case, that this is something useful.” This new work will undoubtedly help build towards that.