
It has been over 60 years since scientists first identified the molecule that we know today as immunoglobulin (Ig) A. IgA is the predominant class of antibody found in human mucus where it is known as secretory (S) IgA. SIgA functions to bind microbes in extracellular regions such as the gut and lungs where it is involved in a variety of poorly understood mechanisms that can promote both pathogen clearance and/or the growth of commensal organisms such as resident gut bacteria.
Unlike most antibody classes, SIgA is a polymeric antibody, typically containing two copies of IgA that assemble in a “tail-to-tail” arrangement together with a protein called the joining-chain (JC) to form dimeric (d) IgA. The dIgA is produced in blood plasma cells and is then transported into the mucosa by a protein called the polymeric Ig-receptor. Interestingly, part of this receptor, called secretory component (SC), remains permanently attached to the SIgA, conferring unique but poorly understood functions.
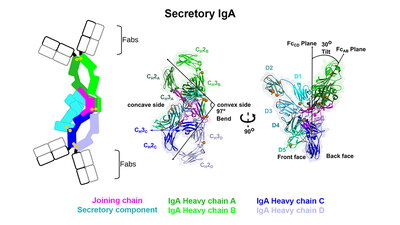
Despite their significance, the molecular structures of dIgA and SIgA have remained elusive for decades, limiting our understanding of an antibody that humans produce daily in gram-quantities. Beth Stadtmueller, assistant professor of biochemistry, and her lab have overcome this challenge by using cryo-electron microscopy to determine the molecular structures of SIgA and its dIgA precursor, which were recently reported in eLife. The structures revealed two IgAs conjoined with the joining-chain protein and bent and tilted with respect to each other, forming distinct concave and convex surfaces.
“One of the observations that we found to be very striking was the bent and tilted arrangement of the two IgAs,” Stadtmueller said. “Many people long assumed that SIgA adopted a relatively linear shape and that the region connecting the two antibodies was flexible, like a hinge. However, we found strong evidence that the two antibodies are rigidly linked in such a way that the angle between them is just over 90 degrees.” Stadtmueller said that comparison of the two structures indicated that the bend between the two antibodies is conferred largely by the joining chain, which adopts an unexpected fold.
Co-lead author Benjamin Parker, a postdoc in the Stadtmueller Lab said, “The joining chain and the two IgAs are effectively woven together and then further stabilized by two joining chain wings, one that wraps around each antibody."
Co-lead author Sonya Kumar Bharathkar, a PhD student in the Stadtmueller Lab, expressed surprise at how the secretory component was associated with the antibody. “It binds mostly on one side of the dIgA and forms a large protrusion from the middle of the complex. We are not yet sure what the functional significance of this is, but we hypothesize that it is involved in binding to host or microbial factors,” Bharathkar said.
The structures suggested that SIgA antibody interactions with antigen (e.g. pathogenic or commensal microbes) and/or with cellular receptors would be influenced by the bent and tilted arrangement of complex components and thus, likely to influence biological functions, Stadtmueller said. To explore this possibility, the group teamed up with Biochemistry and Beckman Institute Professor Emad Tajkhorshid and his graduate student, Nandan Haloi, to computationally model possible positions of SIgA antigen-binding fragments (Fabs). The results revealed a clear preference for Fab positions to be directed toward one side of the SIgA molecule, leaving known receptor-binding sites unobstructed.
How do the new structures impact our understanding of immunology? Stadtmueller said that decades of prior work shed a lot of light on the biological functions of SIgA and revealed structures of some SIgA components; yet without the entire structure, interpreting biological data, understanding functional mechanisms and designing IgA-based therapeutics has remained a challenge. The new IgA structures provide an opportunity to addresses many outstanding questions surrounding SIgA and its functions as a mucosal antibody, she said.
“We have come up with many, many hypotheses as the result of these structures, and testing them is going to be a whole lot of fun,” Stadtmueller said. Another potential long-term outcome of determining the structures is the possibility of engineering IgAs as therapeutic agents. “IgAs have not been widely explored as therapeutic antibodies up to this point and the structures provide insights on how one might go about doing this,” she added.
Stadtmueller emphasized that the use of cryo-electron microscopy was critical to determining the structures. Data for this paper were collected in collaboration with the Caltech Cryo-EM Center in Pasadena, California; however, a new state-of art cryo-EM is currently being installed at UIUC and will open the doors for many labs to solve and analyze cryo-EM structures.
For more information on the Stadtmueller Lab research, visit their website.
