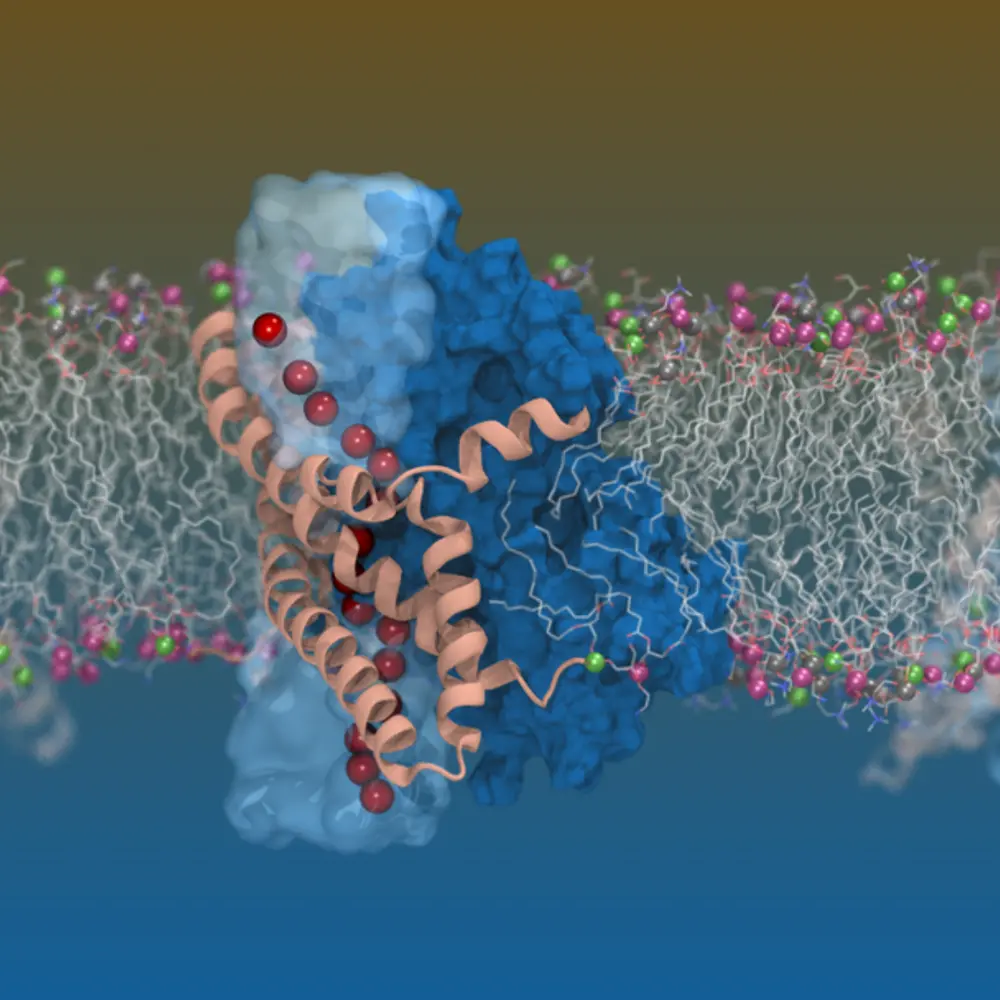
The regulation of glutamate, an excitatory neurotransmitter abundant in the central nervous system, is critical for maintaining normal brain function. Glutamate regulation is achieved via extracellular transport of the amino acid. There are two primary classes of proteins involved in the transport of materials across the membrane: channel proteins, which provide passive pathways for moving materials down their concentration gradient, and transporter proteins, which require an energy input to actively pump materials against the gradient.
University of Illinois professor Emad Tajkhorshid, the J. Woodland Hastings Endowed Chair in Biochemistry, and Shashank Pant, a graduate fellow at the Beckman Institute, studied one particular class of transporters for glutamate regulation—excitatory amino acid transporters (EAATs). EAATs are plasma-membrane-bound transporters that couple glutamate transport to sodium, potassium, and pH gradients. However, in addition to their traditional transporter function, EAATs also possess a channel-like function in their intemediate state. In this state, EAATs activate a reversible and thermodynamically uncoupled conductance of chloride ions.

While researchers have already physiologically characterized this transporter’s channel-like activity, its structure and mechanism were unknown. Using a combination of structural biology with cryogenic electron microscopy (cryo-EM) and computational techniques, an international team of researchers including the Tajkhorshid lab at UIUC and collaborators at University of Sydney was the first to structurally characterize this dual-function transporter in detail. Their research, described in the article, “Glutamate Transporters have a chloride channel with two hydrophobic gates,” was published in Nature.
One of the biggest challenges of this project, researchers said, was experimentally capturing this transient intermediate state, which arises during the transport cycle. They overcame this challenge by using computer simulation of the structural transition of the protein, using the software NAMD to predict the intermediate state structure and design crosslinks that could trap the EAAT chloride-conducting state. This process was extremely laborious and required massive supercomputing time, they said.
Structural biologists typically use cryo-EM to generate a static snapshot of a system. However, this microscopy technique fails to provide insight into the system’s dynamics. Tajkhorshid’s findings are unique as they show the conformational dynamics of this transporter system in a realistic environment. The conformation captured plays a critical role in neurological diseases, such as Alzheimer’s disease and epilepsy.
Tajkhorshid’s research provides a dynamic understanding of the life cycle of EAATs, including all of its conformational states. This will be especially impactful for the development of drugs with high specificity. The Tajkhorshid lab’s novel structural discoveries have significant clinical implications and have provided great insight into the molecular mechanisms of a key transporter protein in the brain.
The National Institutes of Health supported this research.
