Rachel Smith-Bolton, a professor of cell and developmental biology at the University of Illinois, leads an exciting research program on tissue regeneration. Her recent work uses Drosophila as a model to explore the effects of different chromatin modifiers on initiating, spatially controlling, and ending regeneration in coordination with development. In a new publication in Genetics, she and postdoctoral researcher Yuan Tian uncover more mechanisms of regeneration control by showing the roles of two chromatin-remodeling complexes on regeneration in Drosophila through damaging the imaginal disc, the juvenile tissues that build the adult wing during metamorphosis. In particular, they demonstrate the effects of the SWI/SNF BAP and PBAP complexes on Drosophila wing imaginal disc regeneration.
Figuring out how to control regeneration holds immense potential in medicine and beyond, but the tangles of cellular signaling and regulation make this a difficult task. Researchers have been able to identify signals that initiate regeneration in response to tissue damage, but little is understood about how these signals work or why they fail. Regulation of gene expression during this time is also a topic that has eluded researchers. It is also unknown if regeneration genes are all regulated in a similar matter, or if different modification complexes are responsible for chromatin modification at different regeneration genes.

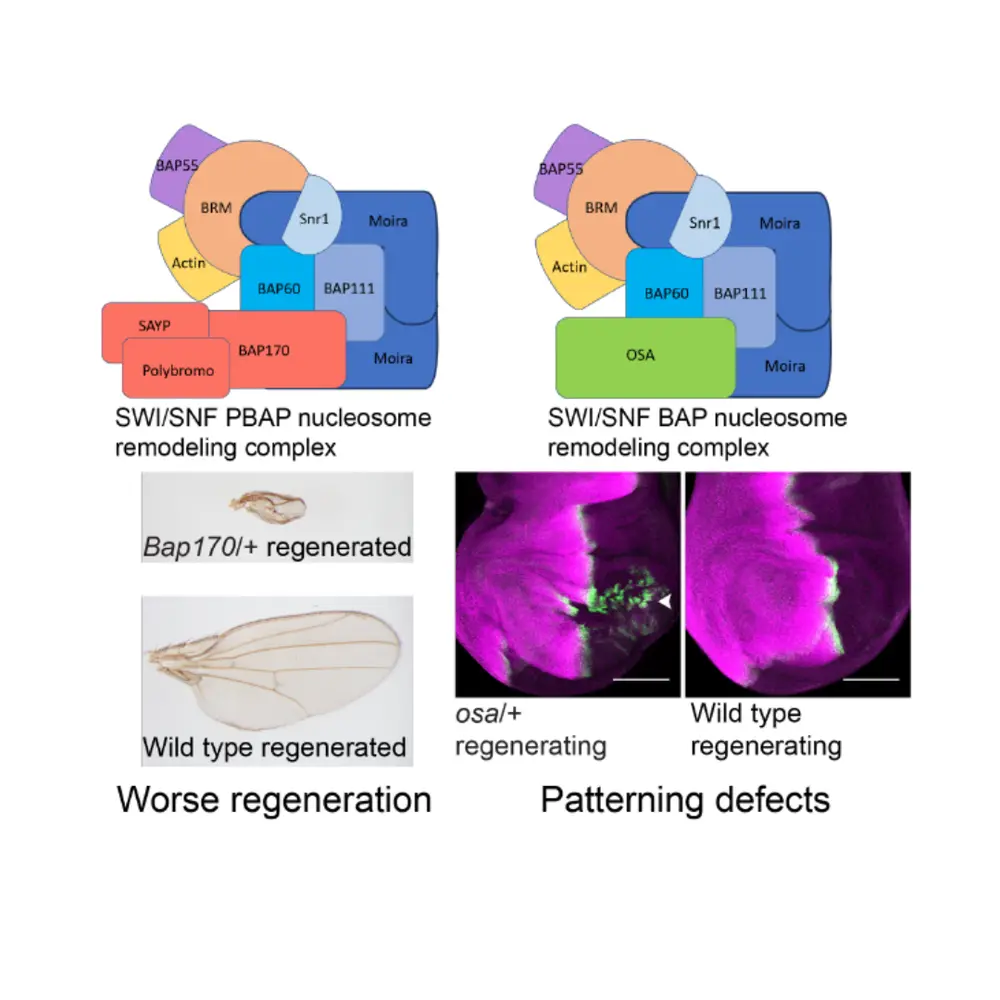
Smith-Bolton and Tian, who received her PhD in cell and developmental biology from Illinois in 2020, have made headway in this area by identifying the mechanisms of controlling regeneration for two chromatin-remodeling complexes: the switch/sucrose non-fermentable (SWI/SNF) complexes Brahma-associated proteins (BAP) and Polybromo-associated proteins (PBAP). While it is known that these complexes play roles in regeneration in a variety of animal models (including Drosophila midgut and mouse skin, liver, and ear), little was previously known about the mechanisms through which the complexes achieve the necessary effects.
Their findings are outlined in the article, “Regulation of growth and cell fate during tissue regeneration by the two SWI/SNF chromatin-remodeling complexes of Drosophila.”
“We know when the tissue gets damaged, there has to be a big change in gene expression. It has to go from what it’s normally doing into damage response and regeneration,” Smith-Bolton said. “One of the big questions that Yuan was tackling was how it makes that switch. Chromatin regulators are a good candidate for how that switch is going to happen.”
In their work, Smith-Bolton and Tian created tightly controllable tissue damage in the Drosophila wing discs using pro-apoptosis genes that can be activated at specific times and in specific tissues. Then, they observed how wing disc regeneration occurred without proper functioning of the BAP and PBAP complexes using mutants and RNAi lines targeting these components. They found that while both complexes act during regeneration, they have very different roles. Drosophila without properly functioning BAP complexes had wing patterning defects after disc damage, with half of the wing pattern appearing as a mirror image of the other half, indicating that this complex plays a role in cell fate and organization of the wing tissue during regeneration.
In Drosophila without a properly functioning PBAP complex, the wing size was much smaller after disc regeneration, demonstrating that this complex plays a role in regenerative growth regulation and synchronization with development. Additionally, the team’s work showed that BAP complex mutants are mispatterned after damage because BAP is needed to protect gene expression against mistakes caused by regeneration signaling.
Smith-Bolton believes that this work has potential for future applications in human regeneration research.
“While it may not be the exact same genes that are going to be important in pushing regeneration in our bodies, the idea that pushing regeneration can cause problems and mistakes is important to think about,” she said. “What do we have to use to stop those mistakes from happening, like the chromatin remodeling complexes that Yuan was working on, to make sure that gene expression doesn’t go haywire when we start pushing regeneration? That's, I think, one of the really important outcomes.”
Added Tian, “Our study of regeneration contributes to the understanding of how tissue regeneration is precisely regulated after tissue damage. This can lead us to achieve proper regeneration through promoting regenerative growth, ensuring correct patterning, and regulating proper ending time instead of scarring or diseases.”
The lab has also identified additional mutations causing abnormal regeneration phenotypes and plans to investigate these mutations in future work.
