Hydrogen peroxide (H2O2) is highly toxic inside most cells and is used by our immune system to kill infecting bacteria. Hydrogen peroxide produces highly reactive hydroxyl radicals via Fenton’s reaction. Reduced iron Fe(II), which is found only inside cells, donates an electron to H2O2 and splits the molecule to form these radicals, making H2O2 a potent biocide.
However, bacterial cells deploy powerful scavenging enzymes, catalases, to eliminate H2O2 before it causes substantial damage. Due to catalases, microbes generally withstand certain high levels of H2O2 without loss in viability. But H2O2 concentrations greater than this threshold can cause severe oxidative damage and immediate lethality. Chromosomal DNA is the main target of oxidative damage to the cell, and this damage can quickly become irreparable.
Interestingly, hydrogen peroxide exhibits synergistic toxicity, meaning typical bacteriostatic (growth-inhibiting) levels of H2O2 can become highly toxic in the presence of other molecules. Professor Andrei Kuzminov and PhD student Pooja Agashe from the Department of Microbiology at the University of Illinois Urbana-Champaign recently explored hydrogen peroxide’s synergistic effects with nitric oxide (NO).

Their findings are reported in the article, “Catalase inhibition by nitric oxide potentiates hydrogen peroxide to trigger catastrophic chromosome fragmentation in Escherichia coli,” in the journal Genetics.
The effects of varying levels of hydrogen peroxide on Escherichia coli was systematically studied in the laboratory of James Imlay, also at the University of Illinois Department of Microbiology. They found that 1 µM of intracellular H2O2 already damages the E. coli chromosome, inactivates iron-containing enzymes and triggers protective transcription response. At the same time, wild-type E coli could still grow in the presence of up to 1 mM of extracellular H2O2. 2-10 mM of extracellular H2O2 was bacteriostatic during short treatments, becoming bactericidal and clastogenic (degrading chromosomal DNA) during long treatments.
These results provided a framework for understanding the synergistic effect of NO on hydrogen peroxide toxicity that interested Agashe and Kuzminov. Nitric oxide production is strongly implicated in host defense against bacterial pathogens. Together, H2O2 and NO are critical for macrophage antimicrobial response and can protect organisms from spontaneous internal infections. NO is a radical species with one unpaired electron. It directly binds to transition metals, including the iron of heme. Cytochrome oxidases of the electron transport chain as well as catalases are examples of heme-containing enzymes in bacterial cells. Imlay and Woodmansee proposed before that the synergistic relationship between NO and H2O2 is due to nitric oxide binding to cytochrome oxidases, thus increasing Fe(II) availability for Fenton’s reaction. Intriguingly, Agashe and Kuzminov were observing additional potentiating effects of NO.
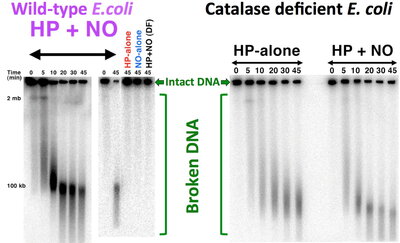
Due to the transient nature of gaseous NO, Agashe and Kuzminov had to put significant effort towards optimizing their experimental conditions, to ensure consistent and accurate release of NO. They confirmed the synergistic relationship between NO and H2O2, but found that it was mostly caused by NO’s inhibition of the heme-containing catalases. Since catalases are the main H2O2 scavengers of the cell, their inhibition caused the intracellular H2O2 concentrations to stabilize.
The resulting high and continuous intracellular H2O2 concentrations induced irreparable chromosome damage, literally breaking chromosome into thousands of fragments (see the Figure). At the same time, this damage was still blocked by iron chelation, which meant that its cause was free cytoplasmic iron, linking results of the current study with the previous results of Imlay and Woodmansee.
Interestingly, in higher eukaryotic cells and even in some bacterial cells, NO can relieve H2O2 toxicity, leading to a so-called "NO paradox." Agashe and Kuzminov also found those conditions: very high H2O2 concentrations kill bacteria rapidly, but NO protects under these conditions, instead of further potentiating the killing. Agashe and Kuzminov speculate that this surprising protective effect of NO could be due to NO complexing intracellular free iron.
The paper by Agashe and Kuzminov provides a framework for understanding the complex relationship between nitric oxide and hydrogen peroxide toxicity, its chromosomal consequences and how our immune cells could use this potent combination against invading bacteria. Currently, Agashe and Kuzminov are comparing the importance of iron cycling versus catalase inhibition in the context of various NO + H2O2 treatments.
This work was supported by a grant from the National Institutes of Health.
