The National Institutes of Health estimates roughly 2.3 million adults and more than 450,000 children in the United States currently live with epilepsy. Although there is a large selection of anti-epileptic drugs, very few exist for neonatal and childhood epilepsy. Furthermore, according to the Epilepsy Foundation, about one-third of adults and approximately 25% of children with epilepsy become resistant to these medications.
Researchers at the University of Illinois Urbana-Champaign are hopeful their findings on the gating mechanisms behind epilepsy-associated potassium channels could provide a foundation for new therapeutic strategies for treating epilepsy, especially in young patients. Their research was recently published in Communications Biology.
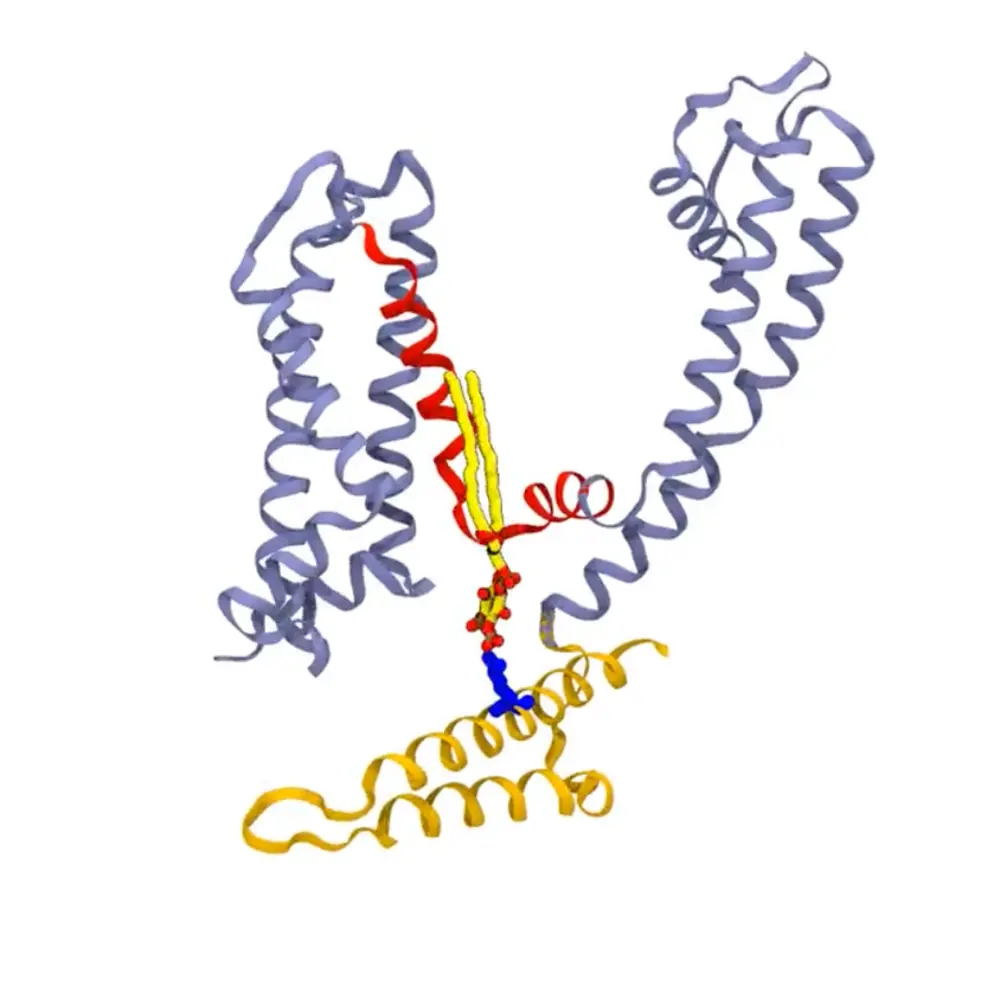
Potassium current produced by neuronal KCNQ/Kv7 channels potently suppresses neuronal firing and excitability. A significant number of mutations exist in Kv7 channel subunits that lead to a wide spectrum of neonatal epilepsies from benign familial neonatal epilepsy (BFNE) to epileptic encephalopathy (EE). EE is often accompanied by drug-resistant seizures, profound developmental delays, and intellectual disabilities.
Hee Jung Chung, professor in the Department of Molecular & Integrative Physiology, jointly supervised the work alongside Emad Tajkhorshid, J. Woodland Hastings Endowed Chair in the Department of Biochemistry, to study how the signaling lipid PIP2 mediates voltage-dependent opening of Kv7 potassium channels by combining all-atom molecular dynamics (MD) simulations on CryoEM structures with electrophysiology measurements.
“Every channel has a canal where the ion goes through,” Chung explains. “But channels are not always open. They have a gate, and for voltage-gated potassium channels, like Kv7, the gate is always closed until the inside of the neuronal membrane becomes electrically positive. This membrane depolarization is sensed by the voltage-sensing domain of the channels, and then, boom, the gate is open.”
Chung says PIP2 binding to this channel is important for it to keep it open. The channel closes when it loses PIP2 binding.
Chung likens the potential therapeutic strategies against epilepsy to keeping the gate of Kv7 channels open.
“So, knowing where PIP2 binds and how PIP2 makes this channel stay open, is directly related to how these channels will suppress neuronal firing,” Chung says.
Previous research published in 2013 by researchers in the Chinese Academy of Sciences and Johns Hopkins School of Medicine identified several potential PIP2 binding regions. However, the study by the Chung and Tajkhorshid labs using simulations of Kv7 channels in lipid bilayers identified many more PIP2 binding residues. Importantly, their studies have revealed that PIP2 binding induces conformational changes and motions related to the gate, which could underlie the molecular mechanism by which PIP2 regulates Kv7 channel opening.

"This was a fascinating example of how lipids, which were considered long to be a passive embedding for proteins, can actively regulate such an important membrane channel," Tajkhorshid says. “Lipid regulation of proteins is yet another dimension in the complex biological organization, which we have just begun to understand. Future research should bring more examples of how this phenomenon significantly affects human health and disease.”
Jiaren Zhang (PhD ’21, Molecular and Cellular Biology), a co-first author and Chung’s former graduate student, says she was intrigued by the new PIP2 binding regions.
“One of the findings I found surprising was how PIP2 molecules come in close contact with the Helix AB linker first, before it ‘walks’ along the C-terminus,” she says.
“Mapping out in-detail the structure of the ion-channels bound to critical signaling molecules such as PIP2 lipids could provide a crucial tool for researchers in understanding how our brain functions and the mechanism behind neurological disorders,” says Shashank Pant (PhD, ’21, biophysics), fellow co-first author and Tajkhorshid’s former graduate student.

Pant is hopeful the maps of the PIP2 binding regions will lay the foundation to explore new therapeutic strategies that can control neuronal Kv7 channels, since current anti-epileptic drugs are ineffective in treating many epilepsy patients with EE.
Retigabine, a Kv7 channel pore opener, has been able to suppress seizures in animal models and humans. Unfortunately, it’s been discontinued as an anti-epileptic drug due to adverse side effects, Chung says. She remains optimistic, though, that drug companies will be able to create improved versions of Retigabine without negative side effects. In addition, Chung and Tajkorshid hope their research will facilitate the development of novel compounds that can promote PIP2 binding to Kv7 channels and thereby sustain their opening.
“Our research is critical because it directs the physician on what kinds of anti-epileptic drugs can be used based on where the mutations are,” Chung says. “It will aid personalized diagnoses and medicine for neonatal epilepsy. That’s why we’re working hard to understand structure-function of epilepsy-associated Kv7 channels.”

