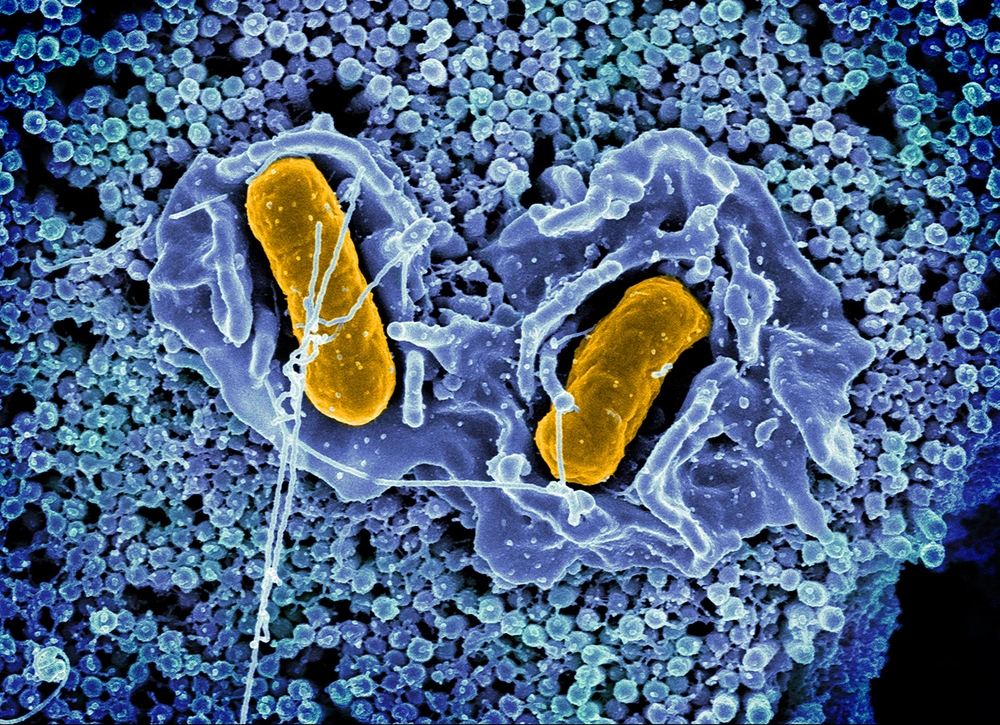
Salmonella is notorious for surviving and replicating in macrophages, which are normally lethal to invading bacteria because of their inhospitable environment. In a new study, researchers have discovered how a system of proteins, called TamAB, helps Salmonella survive under the harsh conditions inside macrophages.
Salmonella is a foodborne pathogen that causes more than a million infections each year in the U.S. Concerningly, it can kill young, old, and immunocompromised individuals. What makes these bacteria especially dangerous is their ability to evade our immune responses.
Macrophages are designed to kill bacteria by spraying them with antibacterial products, exposing them to acidic environments, and withholding magnesium, all of which target the outer layers of the bacteria. Salmonella, however, has evolved mechanisms to survive and grow in this environment.
Under normal conditions, Salmonella uses a complex called Bam to assemble certain proteins that are transported to its outer membrane layer. In previous studies, the group have shown that inside macrophages, the complex is compromised and, as a result, Salmonella depends on the PhoPQ system to sense the environment and orchestrate necessary changes in the outer membrane.
Studies in other bacteria have shown that the TamAB complex performs similar functions to Bam, which led the researchers of the present study to ask whether it might be important in Salmonella. They found that the genes that were responsible for producing TamAB were being controlled by PhoPQ.
“We knew from other studies that TamA was similar to BamA in its structure. When we realized that PhoPQ was controlling this TamAB complex, we hypothesized that the Bam complex struggles in the macrophage and TamA is induced by PhoPQ to help,” said James Slauch, a professor of microbiology.
To test their hypothesis, the researchers first removed TamAB from Salmonella. To their surprise, these mutants were still able to cause an infection in mice. However, when they also crippled the Bam complex, the mutants that lacked TamAB struggled.
The researchers also saw similar results when they recreated the macrophage-like conditions in test tubes and tested the different Salmonella mutants. They observed that mutants that lacked both the Bam and TamAB complexes were sensitive to vancomycin. This result is particularly intriguing because vancomycin is not used to treat Salmonella since it can't cross the outer membrane. This sensitivity suggests that the two complexes have a function in creating or maintaining the outer membrane, although the mechanism is not clear.
“Basically, TamAB helps create favorable conditions for the Bam complex to work but it's indirect,” said Yekaterina Golubeva, a research scientist in the Slauch lab.
It is still unclear what the indirect effect might be. “The problem is that studying the outer membrane is complicated because everything is interconnected. If you mess up the Bam complex, it disrupts additional machineries required for synthesis of the outer membrane. As a result, understanding the contributions of these proteins is difficult,” Slauch said.
Nonetheless, the researchers are now interested in figuring out how TamAB helps. To do so, they will be using suppressor mutants that have accumulated different types of mutations that can help them grow even if their Bam and Tam complexes are defective, providing insights into Salmonella’s outer membrane structure and function.
“There are efforts underway in biotechnology companies that are targeting the Bam complex as a way to treat Salmonella infections,” Slauch said. “Understanding the structure of the outer membrane when Salmonella is in a macrophage can help us understand what will affect its sensitivity to drugs and our results with vancomycin is consistent with that.”
Top photo: Scanning electron micrograph of Salmonella typhimurium invading a human epithelial cell. Photo courtesy of National Institute of Allergy and Infectious Diseases.
The study “TamAB is regulated by PhoPQ and functions in outer membrane homeostasis during Salmonella pathogenesis” was published in the Journal of Bacteriology and can be found at https://doi.org/10.1128/jb.00183-23. The work was funded by the National Institutes of Health.

