In a newly published research, scientists from the School of Molecular & Cellular Biology have reported the discovery of novel proteins that keep Salmonella’s magnesium levels in balance.
Yumi Iwadate, postdoctoral research associate in the Department of Microbiology, and James Slauch, Deb & Tim Paul Chair in Human Infectious Disease & Immunology, authored the article, "The CorC proteins MgpA (YoaE) and CorC protect from excess-magnesium stress and are required for egg white tolerance and virulence in Salmonella" in mBio.
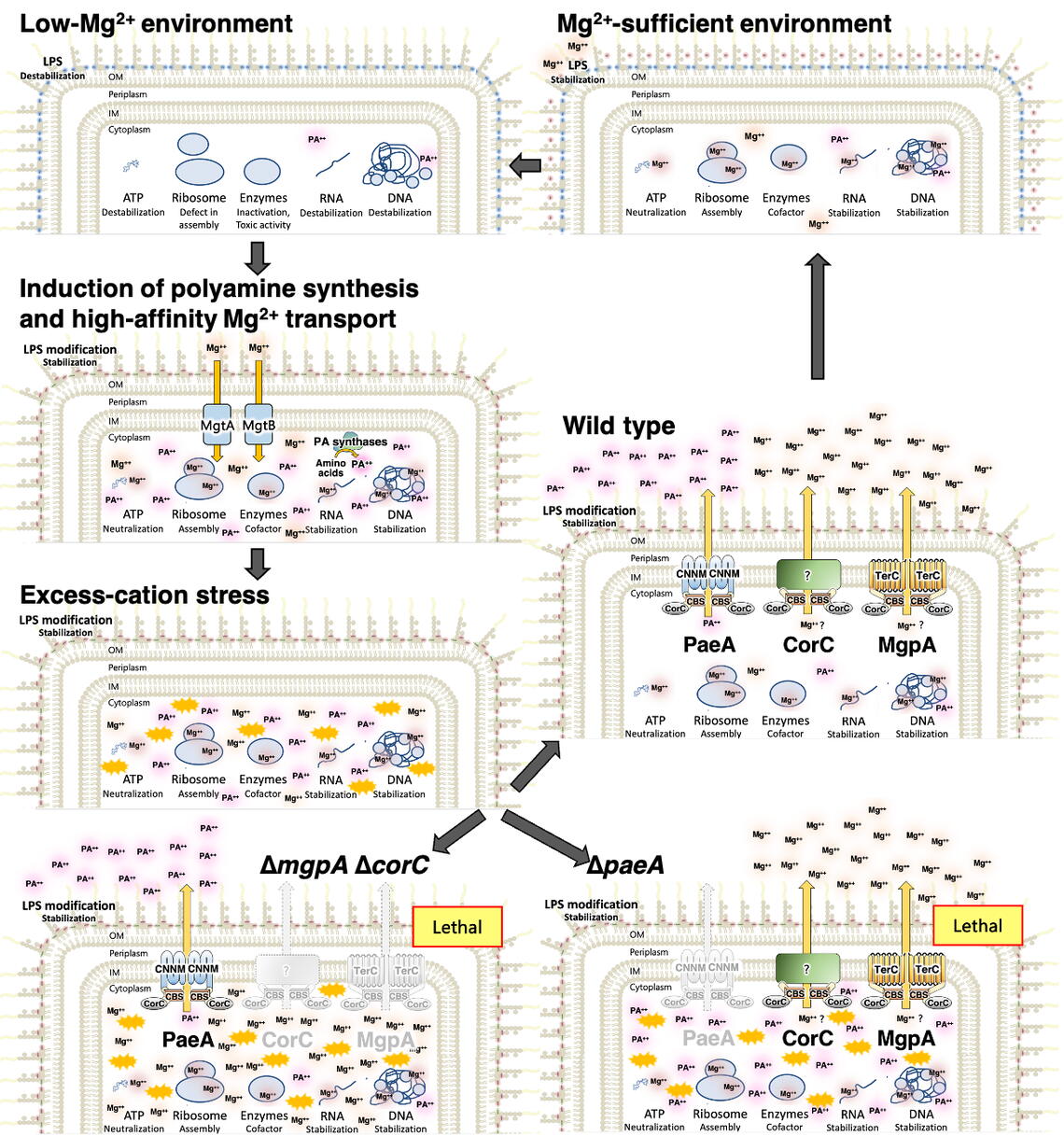
Magnesium is one of the most abundant metals on Earth and within cells, and it is indispensable for life. As a positively charged ion (cation), it stabilizes critical cellular molecules—including ATP, ribosomes, DNA, and RNA—while also acting as a cofactor for many enzymes, according to researchers. Because magnesium levels are tightly tied to cellular growth and protein production, all cells must carefully regulate this element. While the mechanisms of magnesium import are well studied, bacterial magnesium export has remained largely unexplored, even in model organisms like Salmonella and E. coli.
When immune cells called macrophages engulf bacteria, they trap them inside a compartment known as the phagosome and starve them of magnesium to suppress their growth. Yet Salmonella has evolved remarkable ways to survive, causing illnesses that range from food poisoning to life-threatening sepsis. Salmonella manipulates host cells with molecular “weapons” while tolerating the stresses inside the phagosome.
The Slauch Lab has been uncovering these survival strategies for years, more recently showing that balancing cations is a critical part of pathogenesis. Their previous work revealed the role of PaeA, a transporter that reduces excess polyamines during infection.
This new study identifies two additional proteins, CorC and MgpA, which are responsible for reducing excess magnesium, and are essential for Salmonella’s virulence. CorC and MgpA have overlapping functions, thus the maximum defect is seen only when both proteins are missing. This redundancy has made magnesium efflux especially challenging to study. Strikingly, these proteins are also required for the pathogen to survive in egg white, an environment with antimicrobial compounds, but a common source of Salmonella infection.
By illuminating these hidden survival systems, the team’s work not only advances our understanding of basic cell physiology, but also has real-world impact on improving food safety and understanding infectious disease.